Sequential action of JNK genes establishes the embryonic left-right axis
- PMID: 35352808
- PMCID: PMC9148569
- DOI: 10.1242/dev.200136
Sequential action of JNK genes establishes the embryonic left-right axis
Abstract
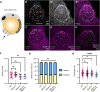
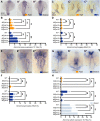
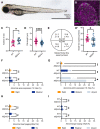
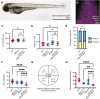
The establishment of the left-right axis is crucial for the placement, morphogenesis and function of internal organs. Left-right specification is proposed to be dependent on cilia-driven fluid flow in the embryonic node. Planar cell polarity (PCP) signalling is crucial for patterning of nodal cilia, yet downstream effectors driving this process remain elusive. We have examined the role of the JNK gene family, a proposed downstream component of PCP signalling, in the development and function of the zebrafish node. We show jnk1 and jnk2 specify length of nodal cilia, generate flow in the node and restrict southpaw to the left lateral plate mesoderm. Moreover, loss of asymmetric southpaw expression does not result in disturbances to asymmetric organ placement, supporting a model in which nodal flow may be dispensable for organ laterality. Later, jnk3 is required to restrict pitx2c expression to the left side and permit correct endodermal organ placement. This work uncovers multiple roles for the JNK gene family acting at different points during left-right axis establishment. It highlights extensive redundancy and indicates JNK activity is distinct from the PCP signalling pathway.
Keywords: Cilia; JNK; Kupffer's vesicle; Laterality; PCP; Zebrafish.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Antic, D., Stubbs, J. L., Suyama, K., Kintner, C., Scott, M. P. and Axelrod, J. D. (2010). Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One 5, e8999. 10.1371/journal.pone.0008999 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

