Endosymbionts Reduce Microbiome Diversity and Modify Host Metabolism and Fecundity in the Planthopper Sogatella furcifera
- PMID: 35353007
- PMCID: PMC9040572
- DOI: 10.1128/msystems.01516-21
Endosymbionts Reduce Microbiome Diversity and Modify Host Metabolism and Fecundity in the Planthopper Sogatella furcifera
Abstract
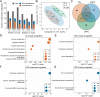
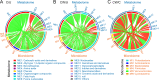
Endosymbionts can strongly affect bacterial microbiota in pests. The white-backed planthopper Sogatella furcifera, a notorious pest in rice, is usually co-infected with Cardinium and Wolbachia, but the effects of these endosymbionts together or individually on the host microbiome and fecundity are unclear. Here, we established three S. furcifera lines (Cardinium and Wolbachia double-infected, Cardinium single-infected, and both-uninfected lines) backcrossed to a common nuclear background and found that single and double infections reduced bacterial diversity and changed bacterial community structure across nymph and adult stages and across adult tissues. The endosymbionts differed in densities between adults and nymphs as well as across adult tissues, with the distribution of Cardinium affected by Wolbachia. Both the single infection and particularly the double infection reduced host fecundity. Lines also differed in levels of metabolites, some of which may influence fecundity (e.g., arginine biosynthesis and nicotinamide metabolism). Cardinium in the single-infected line upregulated metabolic levels, while Wolbachia in the double-infected line appeared to mainly downregulate them. Association analysis pointed to possible connections between various bacteria and differential metabolites. These results reveal that Cardinium by itself and in combination with Wolbachia affect bacterial microbiota and levels of metabolites, with likely effects on host fecundity. Many of the effects of these metabolically limited endosymbionts that are dependent on the hosts may be exerted through manipulation of the microbiome. IMPORTANCE Endosymbionts can profoundly affect the nutrition, immunity, development, and reproduction of insect hosts, but the effects of multiple endosymbiont infections on microbiota and the interaction of these effects with insect host fitness are not well known. By establishing S. furcifera lines with different endosymbiont infection status, we found that Cardinium and the combined Cardinium + Wolbachia infections differentially reduced bacterial diversity as well as changing bacterial community structure and affecting metabolism, which may connect to negative fitness effects of the endosymbionts on their host. These results established the connections between reduced bacterial diversity, decreased fecundity and metabolic responses in S. furcifera.
Keywords: Cardinium and Wolbachia; Sogatella furcifera; bacterial microbiota; correlation; metabolite; microbiome; tissue-specific.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Stable Establishment of Cardinium spp. in the Brown Planthopper Nilaparvata lugens despite Decreased Host Fitness.Appl Environ Microbiol. 2020 Feb 3;86(4):e02509-19. doi: 10.1128/AEM.02509-19. Print 2020 Feb 3. Appl Environ Microbiol. 2020. PMID: 31811033 Free PMC article.
-
Bacterial endosymbiont Cardinium cSfur genome sequence provides insights for understanding the symbiotic relationship in Sogatella furcifera host.BMC Genomics. 2018 Sep 19;19(1):688. doi: 10.1186/s12864-018-5078-y. BMC Genomics. 2018. PMID: 30231855 Free PMC article.
-
Expression of cytoplasmic incompatibility and host fitness effects in field populations of Sogatella furcifera infected with Cardinium.J Econ Entomol. 2012 Dec;105(6):2161-6. doi: 10.1603/ec12268. J Econ Entomol. 2012. PMID: 23356082
-
Fifty shades of bacterial endosymbionts and some of them still remain a mystery: Wolbachia and Cardinium in oribatid mites (Acari: Oribatida).J Invertebr Pathol. 2022 Mar;189:107733. doi: 10.1016/j.jip.2022.107733. Epub 2022 Feb 17. J Invertebr Pathol. 2022. PMID: 35183553 Review.
-
Bacterial reproductive manipulators in rice planthoppers.Arch Insect Biochem Physiol. 2019 Jun;101(2):e21548. doi: 10.1002/arch.21548. Epub 2019 Mar 25. Arch Insect Biochem Physiol. 2019. PMID: 30912174 Review.
Cited by
-
Developmental Shifts in the Microbiome of a Cosmopolitan Pest: Unraveling the Role of Wolbachia and Dominant Bacteria.Insects. 2024 Feb 16;15(2):132. doi: 10.3390/insects15020132. Insects. 2024. PMID: 38392551 Free PMC article.
-
A novel Erwiniaceae gut symbiont modulates gene expression of the intracellular bacterium Cardinium in the stored product mite Tyrophagus putrescentiae.mSphere. 2025 Apr 29;10(4):e0087924. doi: 10.1128/msphere.00879-24. Epub 2025 Mar 24. mSphere. 2025. PMID: 40126013 Free PMC article.
-
Cardinium disrupts Wolbachia-host dynamics in the domestic mite Tyrophagus putrescentiae: evidence from manipulative experiments.mSystems. 2025 May 20;10(5):e0176924. doi: 10.1128/msystems.01769-24. Epub 2025 Apr 18. mSystems. 2025. PMID: 40249197 Free PMC article.
-
Stochastic processes drive divergence of bacterial and fungal communities in sympatric wild insect species despite sharing a common diet.mSphere. 2024 Aug 28;9(8):e0038624. doi: 10.1128/msphere.00386-24. Epub 2024 Aug 6. mSphere. 2024. PMID: 39105581 Free PMC article.
-
Wolbachia Infection Alters the Microbiota of the Invasive Leaf-Miner Liriomyza huidobrensis (Diptera: Agromyzidae).Microorganisms. 2025 Jan 30;13(2):302. doi: 10.3390/microorganisms13020302. Microorganisms. 2025. PMID: 40005669 Free PMC article.
References
-
- Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47. doi:10.1111/j.1365-2435.2008.01442.x. - DOI
-
- Habineza P, Muhammad A, Ji T, Xiao R, Yin X, Hou Y, Shi Z. 2019. The promoting effect of gut microbiota on growth and development of red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: dryophthoridae) by modulating its nutritional metabolism. Front Microbiol 10:1212. doi:10.3389/fmicb.2019.01212. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
