Immune Checkpoint Inhibitor Exposure in Pregnancy: A Scoping Review
- PMID: 35353074
- PMCID: PMC9087868
- DOI: 10.1097/CJI.0000000000000418
Immune Checkpoint Inhibitor Exposure in Pregnancy: A Scoping Review
Abstract
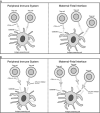
Since their approval, immune checkpoint inhibitors (ICIs) have become the standard of care for multiple malignancies. ICIs enhance tumor destruction by blocking important immunomodulatory pathways that regulate T-cell activation. These pathways include programmed cell death protein-1 and its ligands (programmed cell death protein-1 and programmed death ligand-1, respectively) and cytotoxic T-lymphocyte-associated protein 4. While blocking these pathways can enhance tumor destruction, these pathways are critical for the development of maternal tolerance towards the fetus. Therefore, if ICIs disrupt these immunomodulatory pathways, there could be a maternal immune response against the fetus, as was found in animal studies. With few reported cases of human pregnancy exposure to ICIs, the effects of ICIs on human pregnancy remain largely unknown. Here, we review and summarize the 6 cases of maternal exposure to immunotherapy that have been published before the present study. To add to the evidence, we present a case series of 2 patients who have been exposed to immunotherapy in pregnancy.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
None reported. All authors have declared that there are no financial conflicts of interest with regard to this work.
Figures

References
-
- Burotto M, Gormaz JG, Samtani S, et al. . Viable pregnancy in a patient with metastatic melanoma treated with double checkpoint immunotherapy. Semin Oncol. 2018;45:164–169. - PubMed
-
- Menzer C, Beedgen B, Rom J, et al. . Immunotherapy with ipilimumab plus nivolumab in a stage IV melanoma patient during pregnancy. Eur J Cancer. 2018;104:239–242. - PubMed
-
- Xu W, Moor RJ, Walpole ET, et al. . Pregnancy with successful foetal and maternal outcome in a melanoma patient treated with nivolumab in the first trimester: case report and review of the literature. Melanoma Res. 2019;29:333–337. - PubMed
-
- Bucheit AD, Hardy JT, Szender JB, et al. . Conception and viable twin pregnancy in a patient with metastatic melanoma while treated with CTLA-4 and PD-1 checkpoint inhibition. Melanoma Res. 2020;30:423–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

