Infection-induced lymphatic zippering restricts fluid transport and viral dissemination from skin
- PMID: 35353138
- PMCID: PMC8972184
- DOI: 10.1084/jem.20211830
Infection-induced lymphatic zippering restricts fluid transport and viral dissemination from skin
Abstract
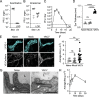
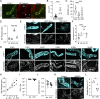
Lymphatic vessels are often considered passive conduits that flush antigenic material, pathogens, and cells to draining lymph nodes. Recent evidence, however, suggests that lymphatic vessels actively regulate diverse processes from antigen transport to leukocyte trafficking and dietary lipid absorption. Here we tested the hypothesis that infection-induced changes in lymphatic transport actively contribute to innate host defense. We demonstrate that cutaneous vaccinia virus infection by scarification activates dermal lymphatic capillary junction tightening (zippering) and lymph node lymphangiogenesis, which are associated with reduced fluid transport and cutaneous viral sequestration. Lymphatic-specific deletion of VEGFR2 prevented infection-induced lymphatic capillary zippering, increased fluid flux out of tissue, and allowed lymphatic dissemination of virus. Further, a reduction in dendritic cell migration to lymph nodes in the absence of lymphatic VEGFR2 associated with reduced antiviral CD8+ T cell expansion. These data indicate that VEGFR2-driven lymphatic remodeling is a context-dependent, active mechanism of innate host defense that limits viral dissemination and facilitates protective, antiviral CD8+ T cell responses.
© 2022 Churchill et al.
Conflict of interest statement
Disclosures: The authors declare no competing financial interests.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

