Dedicated Olfaction and Taste Items do not Improve Psychometric Performance of the SNOT-22
- PMID: 35353381
- PMCID: PMC9544569
- DOI: 10.1002/lary.30120
Dedicated Olfaction and Taste Items do not Improve Psychometric Performance of the SNOT-22
Abstract
Objective: Previous work has shown the chemosensory dysfunction item of the 22-item Sinonasal Outcome Test (SNOT-22) that assesses problems with "taste/smell" has poor psychometric performance compared with other items on the SNOT-22, which we have hypothesized is due to the simultaneous assessment of two different senses. Our aim was to determine whether distinct smell and taste items in the SNOT-22 would improve psychometric performance.
Methods: One hundred and eighty-one CRS patients were recruited and completed the SNOT-22. Additional items querying problems with the senses of "smell" and "taste," using the same response scale and recall period were given to study participants. Item response theory (IRT) was used to determine IRT parameters, including item discrimination, difficulty, and information provided by each SNOT-22 item.
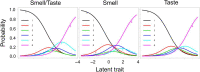
Results: Confirming previous studies, the chemosensory item of the SNOT-22 (reflecting "taste/smell") had poor psychometric performance. Use of a distinct smell or taste item instead of the combined "taste/smell" item did not improve psychometric performance. However, a dedicated smell question resulted in a left shift of threshold parameters, showing that the dedicated smell item better captures moderate CRS disease burden than the original taste/smell item of the SNOT-22, which by virtue of near-identical IRT parameters appears to more greatly reflect problems with taste.
Conclusions: A dedicated smell- or taste-specific item, rather than the combined "taste/smell" item currently in the SNOT-22 does not provide significantly greater psychometric performance. However, a dedicated smell item may better capture moderate CRS disease burden compared with the current chemosensory item on the SNOT-22. Laryngoscope, 132:1644-1651, 2022.
Keywords: CRS; IRT; SNOT-22; chronic rhinosinusitis; graded response model; item response theory; olfaction; quality of life; smell; taste.
© 2022 The Authors. The Laryngoscope published by Wiley Periodicals LLC on behalf of The American Laryngological, Rhinological and Otological Society, Inc.
Figures
Similar articles
-
Item Response Theory for Psychometric Properties of the SNOT-22 (22-Item Sinonasal Outcome Test).Otolaryngol Head Neck Surg. 2022 Mar;166(3):580-588. doi: 10.1177/01945998211018383. Epub 2021 Jun 29. Otolaryngol Head Neck Surg. 2022. PMID: 34182821
-
Exploring possibilities for shortening the 22-item Sino-Nasal Outcome Test (SNOT-22) using item response theory.Int Forum Allergy Rhinol. 2022 Feb;12(2):191-199. doi: 10.1002/alr.22878. Epub 2021 Aug 26. Int Forum Allergy Rhinol. 2022. PMID: 34448367
-
When It's Not Allergic Rhinitis: Clinical Signs to Raise a Patient's Suspicion for Chronic Rhinosinusitis.Otolaryngol Head Neck Surg. 2024 Sep;171(3):708-715. doi: 10.1002/ohn.646. Epub 2024 Jan 31. Otolaryngol Head Neck Surg. 2024. PMID: 38298003
-
The predictive utility of the 22-item sino-nasal outcome test (SNOT-22): A scoping review.Int Forum Allergy Rhinol. 2022 Jan;12(1):83-102. doi: 10.1002/alr.22888. Epub 2021 Sep 28. Int Forum Allergy Rhinol. 2022. PMID: 34585521
-
A Systematic Review and Meta-Analysis of Taste Dysfunction in Chronic Rhinosinusitis.Laryngoscope. 2021 Mar;131(3):482-489. doi: 10.1002/lary.28827. Epub 2020 Jul 1. Laryngoscope. 2021. PMID: 32609889
Cited by
-
[A prospective study of the effect of functional endoscopic sinus surgery on the recovery of olfactory function in patients with chronic rhinosinusitis with nasal polyposis].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023 Jul;37(7):542-549. doi: 10.13201/j.issn.2096-7993.2023.07.007. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023. PMID: 37549946 Free PMC article. Chinese.
-
A scoping review of Rasch analysis and item response theory in otolaryngology: Implications and future possibilities.Laryngoscope Investig Otolaryngol. 2024 Jan 6;9(1):e1208. doi: 10.1002/lio2.1208. eCollection 2024 Feb. Laryngoscope Investig Otolaryngol. 2024. PMID: 38362194 Free PMC article.
-
Unilateral Versus Bilateral Endoscopic Resection of Olfactory Neuroblastoma: Pooled Analysis From Prospective and Retrospective Multicenter Data.Int Forum Allergy Rhinol. 2025 Jun;15(6):585-593. doi: 10.1002/alr.23531. Epub 2025 Jan 15. Int Forum Allergy Rhinol. 2025. PMID: 39811891 Free PMC article.
References
-
- Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Supplement 29):1‐464. - PubMed
-
- Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11:213‐739. - PubMed
-
- Speth MM, Hoehle LP, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. Changes in chronic rhinosinusitis symptoms differentially associate with improvement in general health‐related quality of life. Ann Allergy Asthma Immunol. 2018;121(2):195‐199. - PubMed
-
- Phillips KM, Talat R, Caradonna DS, Gray ST, Sedaghat AR. Quality of life impairment due to chronic rhinosinusitis in asthmatics is mediated by asthma control. Rhinology. 2019;57(6):430‐435. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

