Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model
- PMID: 35353555
- PMCID: PMC8967232
- DOI: 10.1126/sciadv.abk0011
Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model
Abstract
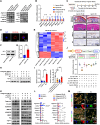
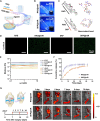
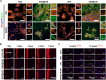
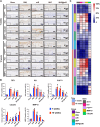
Osteoarthritis (OA) is a common joint disease characterized by progressive loss of cartilage and reduction in lubricating synovial fluid, which lacks effective treatments currently. Here, we propose a hydrogel-based miRNA delivery strategy to rejuvenate impaired cartilage by creating a regenerative microenvironment to mitigate chondrocyte senescence that mainly contributes to cartilage breakdown during OA development. An aging-related miRNA, miR-29b-5p, was first found to be markedly down-regulated in OA cartilage, and their up-regulation suppressed the expression of matrix metalloproteinases and senescence-associated genes (P16INK4a/P21) via ten-eleven-translocation enzyme 1 (TET1). An injectable bioactive self-assembling peptide nanofiber hydrogel was applied to deliver agomir-29b-5p, which was functionalized by conjugating a stem cell-homing peptide SKPPGTSS for endogenous synovial stem cell recruitment simultaneously. Sustained miR-29b-5p delivery and recruitment of synovial stem cells and their subsequent differentiation into chondrocytes led to successful cartilage repair and chondrocyte rejuvenation. This strategy enables miRNA-based therapeutic modality to become a viable alternative for surgery in OA treatment.
Figures



Comment in
-
Targeting senescence in OA.Nat Rev Rheumatol. 2022 Jun;18(6):305. doi: 10.1038/s41584-022-00788-2. Nat Rev Rheumatol. 2022. PMID: 35505106 No abstract available.
References
-
- Hootman J. M., Helmick C. G., Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 54, 226–229 (2006). - PubMed
-
- Hunter D. J., Bierma-Zeinstra S., Osteoarthritis. Lancet 393, 1745–1759 (2019). - PubMed
-
- Stanton H., Rogerson F. M., East C. J., Golub S. B., Lawlor K. E., Meeker C. T., Little C. B., Last K., Farmer P. J., Campbell I. K., Fourie A. M., Fosang A. J., ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

