Biodegradable silicon nanoneedles for ocular drug delivery
- PMID: 35353558
- PMCID: PMC8967230
- DOI: 10.1126/sciadv.abn1772
Biodegradable silicon nanoneedles for ocular drug delivery
Abstract
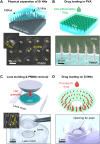
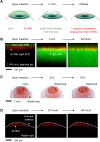
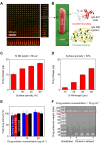
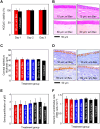
Ocular drug delivery remains a grand challenge due to the complex structure of the eye. Here, we introduce a unique platform of ocular drug delivery through the integration of silicon nanoneedles with a tear-soluble contact lens. The silicon nanoneedles can penetrate into the cornea in a minimally invasive manner and then undergo gradual degradation over the course of months, enabling painless and long-term sustained delivery of ocular drugs. The tear-soluble contact lens can fit a variety of corneal sizes and then quickly dissolve in tear fluid within a minute, enabling an initial burst release of anti-inflammatory drugs. We demonstrated the utility of this platform in effectively treating a chronic ocular disease, such as corneal neovascularization, in a rabbit model without showing a notable side effect over current standard therapies. This platform could also be useful in treating other chronic ocular diseases.
Figures


Similar articles
-
Long-Term Diabetic Retinopathy Treatment Using Silicon Nanoneedles.Small. 2025 Mar;21(9):e2410166. doi: 10.1002/smll.202410166. Epub 2025 Feb 5. Small. 2025. PMID: 39910900
-
Steroid-eluting contact lenses for corneal and intraocular inflammation.Acta Biomater. 2020 Oct 15;116:149-161. doi: 10.1016/j.actbio.2020.08.013. Epub 2020 Aug 16. Acta Biomater. 2020. PMID: 32814140 Free PMC article.
-
Inner layer-embedded contact lenses for pH-triggered controlled ocular drug delivery.Eur J Pharm Biopharm. 2018 Jul;128:220-229. doi: 10.1016/j.ejpb.2018.04.017. Epub 2018 May 3. Eur J Pharm Biopharm. 2018. PMID: 29730260
-
Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses.Adv Colloid Interface Sci. 2016 Jul;233:139-154. doi: 10.1016/j.cis.2015.08.002. Epub 2015 Aug 14. Adv Colloid Interface Sci. 2016. PMID: 26318359 Review.
-
2. Contact lens care and ocular surface homeostasis.Cont Lens Anterior Eye. 2013 Jan 15;36 Suppl 1:S9-13. doi: 10.1016/S1367-0484(13)60004-1. Cont Lens Anterior Eye. 2013. PMID: 23347575 Review.
Cited by
-
Microneedles for in situ tissue regeneration.Mater Today Bio. 2023 Feb 11;19:100579. doi: 10.1016/j.mtbio.2023.100579. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36880084 Free PMC article. Review.
-
Smart Contact Lenses as Wearable Ophthalmic Devices for Disease Monitoring and Health Management.Chem Rev. 2023 Oct 11;123(19):11488-11558. doi: 10.1021/acs.chemrev.3c00290. Epub 2023 Sep 25. Chem Rev. 2023. PMID: 37748126 Free PMC article. Review.
-
Application of Convergent Science and Technology toward Ocular Disease Treatment.Pharmaceuticals (Basel). 2023 Mar 16;16(3):445. doi: 10.3390/ph16030445. Pharmaceuticals (Basel). 2023. PMID: 36986546 Free PMC article. Review.
-
Microneedles for controlled and sustained intraocular drug delivery.NPG Asia Mater. 2025;17(1):33. doi: 10.1038/s41427-025-00614-7. Epub 2025 Aug 22. NPG Asia Mater. 2025. PMID: 40860929 Free PMC article. Review.
-
Mechanically-Guided 3D Assembly for Architected Flexible Electronics.Chem Rev. 2023 Sep 27;123(18):11137-11189. doi: 10.1021/acs.chemrev.3c00335. Epub 2023 Sep 7. Chem Rev. 2023. PMID: 37676059 Free PMC article. Review.
References
-
- Zhang K., Zhang L., Weinreb R. N., Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 11, 541–559 (2012). - PubMed
-
- Clark A. F., Yorio T., Ophthalmic drug discovery. Nat. Rev. Drug Discov. 2, 448–459 (2003). - PubMed
-
- Ghosh J. G., Nguyen A. A., Bigelow C. E., Poor S., Qiu Y., Rangaswamy N., Ornberg R., Jackson B., Mak H., Ezell T., Kenanova V., de La Cruz E., Carrion A., Etemad-Gilbertson B., Caro R. G., Zhu K., George V., Bai J., Sharma-Nahar R., Shen S., Wang Y., Subramanian K. K., Fassbender E., Maker M., Hanks S., Vrouvlianis J., Leehy B., Long D., Prentiss M., Kansara V., Jaffee B., Dryja T. P., Roguska M., Long-acting protein drugs for the treatment of ocular diseases. Nat. Commun. 8, 14837 (2017). - PMC - PubMed
-
- Yuan X., Marcano D. C., Shin C. S., Hua X., Isenhart L. C., Pflugfelder S. C., Acharya G., Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano 9, 1749–1758 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

