Asynchronous nuclear cycles in multinucleated Plasmodium falciparum facilitate rapid proliferation
- PMID: 35353560
- PMCID: PMC8967237
- DOI: 10.1126/sciadv.abj5362
Asynchronous nuclear cycles in multinucleated Plasmodium falciparum facilitate rapid proliferation
Abstract
Malaria-causing parasites proliferate within erythrocytes through schizogony, forming multinucleated stages before cellularization. Nuclear multiplication does not follow a strict geometric 2n progression, and each proliferative cycle produces a variable number of progeny. Here, by tracking nuclei and DNA replication, we show that individual nuclei replicate their DNA at different times, despite residing in a shared cytoplasm. Extrapolating from experimental data using mathematical modeling, we provide strong indication that a limiting factor exists, which slows down the nuclear multiplication rate. Consistent with this prediction, our data show that temporally overlapping DNA replication events were significantly slower than partially overlapping or nonoverlapping events. Our findings suggest the existence of evolutionary pressure that selects for asynchronous DNA replication, balancing available resources with rapid pathogen proliferation.
Figures


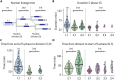

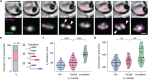
Comment in
-
Hand-in-hand advances in microscopy and Plasmodium nuclear biology.Trends Parasitol. 2022 Jun;38(6):421-423. doi: 10.1016/j.pt.2022.03.007. Epub 2022 Apr 18. Trends Parasitol. 2022. PMID: 35450787
Similar articles
-
DNA replication dynamics during erythrocytic schizogony in the malaria parasites Plasmodium falciparum and Plasmodium knowlesi.PLoS Pathog. 2022 Jun 22;18(6):e1010595. doi: 10.1371/journal.ppat.1010595. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35731838 Free PMC article.
-
Plasmodium falciparum CRK4 links early mitotic events to the onset of S-phase during schizogony.mBio. 2023 Aug 31;14(4):e0077923. doi: 10.1128/mbio.00779-23. Epub 2023 Jun 22. mBio. 2023. PMID: 37345936 Free PMC article.
-
Plasmodium falciparum CRK4 directs continuous rounds of DNA replication during schizogony.Nat Microbiol. 2017 Feb 17;2:17017. doi: 10.1038/nmicrobiol.2017.17. Nat Microbiol. 2017. PMID: 28211852 Free PMC article.
-
Plasmodium schizogony, a chronology of the parasite's cell cycle in the blood stage.PLoS Pathog. 2023 Mar 2;19(3):e1011157. doi: 10.1371/journal.ppat.1011157. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36862652 Free PMC article. Review.
-
How Many Is Enough? - Challenges of Multinucleated Cell Division in Malaria Parasites.Front Cell Infect Microbiol. 2021 May 7;11:658616. doi: 10.3389/fcimb.2021.658616. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34026661 Free PMC article. Review.
Cited by
-
DNA replication dynamics during erythrocytic schizogony in the malaria parasites Plasmodium falciparum and Plasmodium knowlesi.PLoS Pathog. 2022 Jun 22;18(6):e1010595. doi: 10.1371/journal.ppat.1010595. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35731838 Free PMC article.
-
An extended DNA-free intranuclear compartment organizes centrosome microtubules in malaria parasites.Life Sci Alliance. 2021 Sep 17;4(11):e202101199. doi: 10.26508/lsa.202101199. Print 2021 Nov. Life Sci Alliance. 2021. PMID: 34535568 Free PMC article.
-
Progeny counter mechanism in malaria parasites is linked to extracellular resources.PLoS Pathog. 2023 Dec 5;19(12):e1011807. doi: 10.1371/journal.ppat.1011807. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38051755 Free PMC article.
-
Fluorescent Nitrogen-Doped Carbon Dots for Label Live Elder Blood-Stage Plasmodium falciparum through New Permeability Pathways.Molecules. 2022 Jun 29;27(13):4163. doi: 10.3390/molecules27134163. Molecules. 2022. PMID: 35807422 Free PMC article.
-
Same, same but different: Exploring Plasmodium cell division during liver stage development.PLoS Pathog. 2023 Mar 30;19(3):e1011210. doi: 10.1371/journal.ppat.1011210. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36996035 Free PMC article. Review.
References
-
- Adl S. M., Bass D., Lane C. E., Lukeš J., Schoch C. L., Smirnov A., Agatha S., Berney C., Brown M. W., Burki F., Cárdenas P., Čepička I., Chistyakova L., Campo J., Dunthorn M., Edvardsen B., Eglit Y., Guillou L., Hampl V., Heiss A. A., Hoppenrath M., James T. Y., Karnkowska A., Karpov S., Kim E., Kolisko M., Kudryavtsev A., Lahr D. J. G., Lara E., Gall L. L., Lynn D. H., Mann D. G., Massana R., Mitchell E. A. D., Morrow C., Park J. S., Pawlowski J. W., Powell M. J., Richter D. J., Rueckert S., Shadwick L., Shimano S., Spiegel F. W., Torruella G., Youssef N., Zlatogursky V., Zhang Q., Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 66, 4–119 (2019). - PMC - PubMed
-
- Berger F., Endosperm development. Curr. Opin. Plant Biol. 2, 28–32 (1999). - PubMed
-
- Boyle W. J., Simonet W. S., Lacey D. L., Osteoclast differentiation and activation. Nature 423, 337–342 (2003). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

