Neuronal C/EBPβ/AEP pathway shortens life span via selective GABAnergic neuronal degeneration by FOXO repression
- PMID: 35353567
- PMCID: PMC8967231
- DOI: 10.1126/sciadv.abj8658
Neuronal C/EBPβ/AEP pathway shortens life span via selective GABAnergic neuronal degeneration by FOXO repression
Abstract
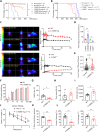
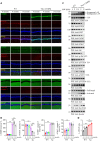
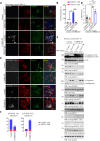
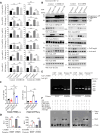
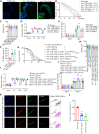
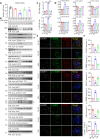
The age-related cognitive decline of normal aging is exacerbated in neurodegenerative diseases including Alzheimer's disease (AD). However, it remains unclear whether age-related cognitive regulators in AD pathologies contribute to life span. Here, we show that C/EBPβ, an Aβ and inflammatory cytokine-activated transcription factor that promotes AD pathologies via activating asparagine endopeptidase (AEP), mediates longevity in a gene dose-dependent manner in neuronal C/EBPβ transgenic mice. C/EBPβ selectively triggers inhibitory GABAnergic neuronal degeneration by repressing FOXOs and up-regulating AEP, leading to aberrant neural excitation and cognitive dysfunction. Overexpression of CEBP-2 or LGMN-1 (AEP) in Caenorhabditis elegans neurons but not muscle stimulates neural excitation and shortens life span. CEBP-2 or LGMN-1 reduces daf-2 mutant-elongated life span and diminishes daf-16-induced longevity. C/EBPβ and AEP are lower in humans with extended longevity and inversely correlated with REST/FOXO1. These findings demonstrate a conserved mechanism of aging that couples pathological cognitive decline to life span by the neuronal C/EBPβ/AEP pathway.
Figures
References
-
- Apfeld J., Kenyon C., Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809 (1999). - PubMed
-
- Alcedo J., Kenyon C., Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45–55 (2004). - PubMed
-
- Bishop N. A., Guarente L., Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549 (2007). - PubMed
-
- Wolkow C. A., Kimura K. D., Lee M. S., Ruvkun G., Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290, 147–150 (2000). - PubMed
-
- Broughton S. J., Piper M. D. W., Ikeya T., Bass T. M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D. J., Leevers S. J., Partridge L., Longer lifespan, altered metabolism, and stress resistance in drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U.S.A. 102, 3105–3110 (2005). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

