Formation of cellular close-ended tunneling nanotubes through mechanical deformation
- PMID: 35353579
- PMCID: PMC8967236
- DOI: 10.1126/sciadv.abj3995
Formation of cellular close-ended tunneling nanotubes through mechanical deformation
Abstract
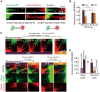
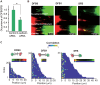
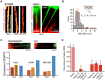
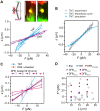
Membrane nanotubes or tunneling nanotubes (TNTs) that connect cells have been recognized as a previously unidentified pathway for intercellular transport between distant cells. However, it is unknown how this delicate structure, which extends over tens of micrometers and remains robust for hours, is formed. Here, we found that a TNT develops from a double filopodial bridge (DFB) created by the physical contact of two filopodia through helical deformation of the DFB. The transition of a DFB to a close-ended TNT is most likely triggered by disruption of the adhesion of two filopodia by mechanical energy accumulated in a twisted DFB when one of the DFB ends is firmly attached through intercellular cadherin-cadherin interactions. These studies pinpoint the mechanistic questions about TNTs and elucidate a formation mechanism.
Figures

References
-
- Rustom A., Saffrich R., Markovic I., Walther P., Gerdes H. H., Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010 (2004). - PubMed
-
- Önfelt B., Nedvetzki S., Benninger R. K. P., Purbhoo M. A., Sowinski S., Hume A. N., Seabra M. C., Neil M. A. A., French P. M. W., Davis D. M., Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177, 8476–8483 (2006). - PubMed
-
- Önfelt B., Nedvetzki S., Yanagi K., Davis D. M., Cutting edge: Membrane nanotubes connect immune cells. J. Immunol. 173, 1511–1513 (2004). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

