Early Outpatient Treatment for Covid-19 with Convalescent Plasma
- PMID: 35353960
- PMCID: PMC9006786
- DOI: 10.1056/NEJMoa2119657
Early Outpatient Treatment for Covid-19 with Convalescent Plasma
Abstract
Background: Polyclonal convalescent plasma may be obtained from donors who have recovered from coronavirus disease 2019 (Covid-19). The efficacy of this plasma in preventing serious complications in outpatients with recent-onset Covid-19 is uncertain.
Methods: In this multicenter, double-blind, randomized, controlled trial, we evaluated the efficacy and safety of Covid-19 convalescent plasma, as compared with control plasma, in symptomatic adults (≥18 years of age) who had tested positive for severe acute respiratory syndrome coronavirus 2, regardless of their risk factors for disease progression or vaccination status. Participants were enrolled within 8 days after symptom onset and received a transfusion within 1 day after randomization. The primary outcome was Covid-19-related hospitalization within 28 days after transfusion.
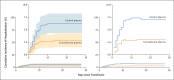
Results: Participants were enrolled from June 3, 2020, through October 1, 2021. A total of 1225 participants underwent randomization, and 1181 received a transfusion. In the prespecified modified intention-to-treat analysis that included only participants who received a transfusion, the primary outcome occurred in 17 of 592 participants (2.9%) who received convalescent plasma and 37 of 589 participants (6.3%) who received control plasma (absolute risk reduction, 3.4 percentage points; 95% confidence interval, 1.0 to 5.8; P = 0.005), which corresponded to a relative risk reduction of 54%. Evidence of efficacy in vaccinated participants cannot be inferred from these data because 53 of the 54 participants with Covid-19 who were hospitalized were unvaccinated and 1 participant was partially vaccinated. A total of 16 grade 3 or 4 adverse events (7 in the convalescent-plasma group and 9 in the control-plasma group) occurred in participants who were not hospitalized.
Conclusions: In participants with Covid-19, most of whom were unvaccinated, the administration of convalescent plasma within 9 days after the onset of symptoms reduced the risk of disease progression leading to hospitalization. (Funded by the Department of Defense and others; CSSC-004 ClinicalTrials.gov number, NCT04373460.).
Copyright © 2022 Massachusetts Medical Society.
Figures


Comment in
-
Convalescent Plasma for Covid-19 - Making Sense of the Inconsistencies.N Engl J Med. 2022 May 5;386(18):1753-1754. doi: 10.1056/NEJMe2204332. N Engl J Med. 2022. PMID: 35507487 Free PMC article. No abstract available.
-
Convalescent Plasma for Covid-19.N Engl J Med. 2022 Sep 8;387(10):955. doi: 10.1056/NEJMc2208338. N Engl J Med. 2022. PMID: 36069882 No abstract available.
-
Convalescent Plasma for Covid-19.N Engl J Med. 2022 Sep 8;387(10):955. doi: 10.1056/NEJMc2208338. N Engl J Med. 2022. PMID: 36069883 No abstract available.
References
-
- Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021;385:1941-1950. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
