Effect of Early Treatment with Ivermectin among Patients with Covid-19
- PMID: 35353979
- PMCID: PMC9006771
- DOI: 10.1056/NEJMoa2115869
Effect of Early Treatment with Ivermectin among Patients with Covid-19
Abstract
Background: The efficacy of ivermectin in preventing hospitalization or extended observation in an emergency setting among outpatients with acutely symptomatic coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is unclear.
Methods: We conducted a double-blind, randomized, placebo-controlled, adaptive platform trial involving symptomatic SARS-CoV-2-positive adults recruited from 12 public health clinics in Brazil. Patients who had had symptoms of Covid-19 for up to 7 days and had at least one risk factor for disease progression were randomly assigned to receive ivermectin (400 μg per kilogram of body weight) once daily for 3 days or placebo. (The trial also involved other interventions that are not reported here.) The primary composite outcome was hospitalization due to Covid-19 within 28 days after randomization or an emergency department visit due to clinical worsening of Covid-19 (defined as the participant remaining under observation for >6 hours) within 28 days after randomization.
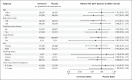
Results: A total of 3515 patients were randomly assigned to receive ivermectin (679 patients), placebo (679), or another intervention (2157). Overall, 100 patients (14.7%) in the ivermectin group had a primary-outcome event, as compared with 111 (16.3%) in the placebo group (relative risk, 0.90; 95% Bayesian credible interval, 0.70 to 1.16). Of the 211 primary-outcome events, 171 (81.0%) were hospital admissions. Findings were similar to the primary analysis in a modified intention-to-treat analysis that included only patients who received at least one dose of ivermectin or placebo (relative risk, 0.89; 95% Bayesian credible interval, 0.69 to 1.15) and in a per-protocol analysis that included only patients who reported 100% adherence to the assigned regimen (relative risk, 0.94; 95% Bayesian credible interval, 0.67 to 1.35). There were no significant effects of ivermectin use on secondary outcomes or adverse events.
Conclusions: Treatment with ivermectin did not result in a lower incidence of medical admission to a hospital due to progression of Covid-19 or of prolonged emergency department observation among outpatients with an early diagnosis of Covid-19. (Funded by FastGrants and the Rainwater Charitable Foundation; TOGETHER ClinicalTrials.gov number, NCT04727424.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Ivermectin Treatment for Covid-19.N Engl J Med. 2022 Dec 15;387(24):e66. doi: 10.1056/NEJMc2207995. N Engl J Med. 2022. PMID: 36516101 No abstract available.
-
Ivermectin Treatment for Covid-19.N Engl J Med. 2022 Dec 15;387(24):e66. doi: 10.1056/NEJMc2207995. N Engl J Med. 2022. PMID: 36516102 No abstract available.
-
Ivermectin Treatment for Covid-19.N Engl J Med. 2022 Dec 15;387(24):e66. doi: 10.1056/NEJMc2207995. N Engl J Med. 2022. PMID: 36516103 No abstract available.
-
Ivermectin Treatment for Covid-19. Reply.N Engl J Med. 2022 Dec 15;387(24):e66. doi: 10.1056/NEJMc2207995. N Engl J Med. 2022. PMID: 36516104 No abstract available.
References
-
- Forrester SG, Prichard RK, Beech RN. A glutamate-gated chloride channel subunit from Haemonchus contortus: expression in a mammalian cell line, ligand binding, and modulation of anthelmintic binding by glutamate. Biochem Pharmacol 2002;63:1061-1068. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
