Polygenic risk for prostate cancer: Decreasing relative risk with age but little impact on absolute risk
- PMID: 35353984
- PMCID: PMC9118111
- DOI: 10.1016/j.ajhg.2022.03.008
Polygenic risk for prostate cancer: Decreasing relative risk with age but little impact on absolute risk
Abstract
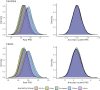
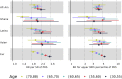
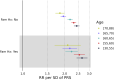
Polygenic risk scores (PRSs) for a variety of diseases have recently been shown to have relative risks that depend on age, and genetic relative risks decrease with increasing age. A refined understanding of the age dependency of PRSs for a disease is important for personalized risk predictions and risk stratification. To further evaluate how the PRS relative risk for prostate cancer depends on age, we refined analyses for a validated PRS for prostate cancer by using 64,274 prostate cancer cases and 46,432 controls of diverse ancestry (82.8% European, 9.8% African American, 3.8% Latino, 2.8% Asian, and 0.8% Ghanaian). Our strategy applied a novel weighted proportional hazards model to case-control data to fully utilize age to refine how the relative risk decreased with age. We found significantly greater relative risks for younger men (age 30-55 years) compared with older men (70-88 years) for both relative risk per standard deviation of the PRS and dichotomized according to the upper 90th percentile of the PRS distribution. For the largest European ancestral group that could provide reliable resolution, the log-relative risk decreased approximately linearly from age 50 to age 75. Despite strong evidence of age-dependent genetic relative risk, our results suggest that absolute risk predictions differed little from predictions that assumed a constant relative risk over ages, from short-term to long-term predictions, simplifying implementation of risk discussions into clinical practice.
Keywords: absolute risk prediction; genetic relative risk; weighted Cox regression.
Copyright © 2022 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

