Sequential sampling from memory underlies action selection during abstract decision-making
- PMID: 35354066
- PMCID: PMC9090972
- DOI: 10.1016/j.cub.2022.03.014
Sequential sampling from memory underlies action selection during abstract decision-making
Abstract
The study of perceptual decision-making in monkeys has provided insights into the process by which sensory evidence is integrated toward a decision. When monkeys make decisions with the knowledge of the motor actions the decisions bear upon, the process of evidence integration is instantiated by neurons involved in the selection of said actions. It is less clear how monkeys make decisions when unaware of the actions required to communicate their choice-what we refer to as "abstract" decisions. We investigated this by training monkeys to associate the direction of motion of a noisy random-dot display with the color of two targets. Crucially, the targets were displayed at unpredictable locations after the motion stimulus was extinguished. We found that the monkeys postponed decision formation until the targets were revealed. Neurons in the parietal association area LIP represented the integration of evidence leading to a choice, but as the stimulus was no longer visible, the samples of evidence must have been retrieved from short-term memory. Our results imply that when decisions are temporally unyoked from the motor actions they bear upon, decision formation is protracted until they can be framed in terms of motor actions.
Keywords: decision making, abstraction, short-term memory, area LIP, macaque monkey.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests MNS is a member of the advisory board of Current Biology. The other authors declare no competing interests.
Figures
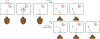


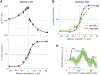
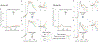
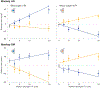

Comment in
-
Cognitive neuroscience: Mental replay in monkeys.Curr Biol. 2022 May 9;32(9):R430-R432. doi: 10.1016/j.cub.2022.03.061. Curr Biol. 2022. PMID: 35537397
-
Accumulating evidence by sampling from temporally organized memory.Learn Behav. 2023 Dec;51(4):351-352. doi: 10.3758/s13420-022-00569-7. Epub 2023 Jan 17. Learn Behav. 2023. PMID: 36650395
References
-
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex [Journal Article]. Science. 2001;291(5502):312–6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11209083. - PubMed
-
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex [Journal Article]. Nature. 2006;443(7107):85–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16936716. - PubMed
-
- Seger CA, Miller EK. Category learning in the brain [Journal Article]. Annu Rev Neurosci. 2010;33:203–19. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20572771. - PMC - PubMed
-
- Goodwin SJ, Blackman RK, Sakellaridi S, Chafee MV. Executive control over cognition: stronger and earlier rule-based modulation of spatial category signals in prefrontal cortex relative to parietal cortex [Journal Article]. J Neurosci. 2012;32(10):3499–515. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22399773. - PMC - PubMed
-
- Tenenbaum JB, Kemp C, Griffiths TL, Goodman ND. How to grow a mind: statistics, structure, and abstraction [Journal Article]. Science. 2011;331(6022):1279–85. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21393536. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

