Demystifying mRNA vaccines: an emerging platform at the forefront of cryptic diseases
- PMID: 35354425
- PMCID: PMC8973339
- DOI: 10.1080/15476286.2022.2055923
Demystifying mRNA vaccines: an emerging platform at the forefront of cryptic diseases
Abstract
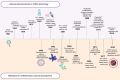
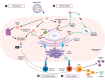
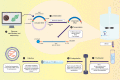
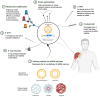
Messenger RNA (mRNA) vaccines have been studied for decades, but only recently, during the COVID-19 pandemic, has the technology garnered noteworthy attention. In contrast to traditional vaccines, mRNA vaccines elicit a more balanced immune response, triggering both humoral and cellular components of the adaptive immune system. However, some inherent hurdles associated with stability, immunogenicity, in vivo delivery, along with the novelty of the technology, have generated scepticism in the adoption of mRNA vaccines. Recent developments have pushed to bypass these issues and the approval of mRNA-based vaccines to combat COVID-19 has further highlighted the feasibility, safety, efficacy, and rapid development potential of this platform, thereby pushing it to the forefront of emerging therapeutics. This review aims to demystify mRNA vaccines, delineating the evolution of the technology which has emerged as a timely solution to COVID-19 and exploring the immense potential it offers as a prophylactic option for other cryptic diseases.
Keywords: COVID-19; IVT-mRNA; in vivo delivery of mRNA; mRNA vaccine; mRNA vaccine for infectious disease; nucleoside modified mRNA; vaccine efficacy; vaccine storage.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
