Immune responses in the irritable bowel syndromes: time to consider the small intestine
- PMID: 35354471
- PMCID: PMC8969236
- DOI: 10.1186/s12916-022-02301-8
Immune responses in the irritable bowel syndromes: time to consider the small intestine
Abstract
Background: Irritable bowel syndrome (IBS) is considered a disorder of gut-brain interaction (DGBI), presenting as chronic abdominal pain and altered defaecation. Symptoms are often food related. Much work in the field has focused on identifying physiological, immune and microbial abnormalities in the colon of patients; however, evidence of small intestinal immune activation and microbial imbalance has been reported in small studies. The significance of such findings has been largely underappreciated despite a growing body of work implicating small intestinal homeostatic imbalance in the pathogenesis of DGBIs.
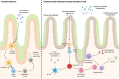
Main text: Small intestinal mechanosensation is a characteristic feature of IBS. Furthermore, altered small intestinal barrier functions have been demonstrated in IBS patients with the diarrhoea-predominant subtype. Small intestinal bacterial overgrowth and increased populations of small intestinal mast cells are frequently associated with IBS, implicating microbial imbalance and low-grade inflammation in the pathogenesis of IBS. Furthermore, reports of localised food hypersensitivity responses in IBS patients implicate the small intestine as the site of immune-microbial-food interactions.
Conclusions: Given the association of IBS symptoms with food intake in a large proportion of patients and the emerging evidence of immune activation in these patients, the current literature suggests the pathogenesis of IBS is not limited to the colon but rather may involve dysfunction of the entire intestinal tract. It remains unclear if regional variation in IBS pathology explains the various symptom phenotypes and further work should consider the intestinal tract as a whole to answer this question.
Keywords: Disorders of gut-brain interaction; Functional gastrointestinal disorders; Immune; Irritable bowel syndrome; Small intestine.
© 2022. The Author(s).
Conflict of interest statement
GLB declares that she has no competing interests.
NJT: Dr. Talley reports non-financial support from HVN National Science Challenge NZ, personal fees from Aviro Health (Digestive health) (2019), Anatara Life Sciences, Brisbane (2019), Allakos (gastric eosinophilic disease) (2021), Bayer [IBS] (2020), Danone (Probiotic) (2018), Planet Innovation (Gas capsule IBS) (2020), Takeda, Japan (gastroparesis) (2019), twoXAR (2019) (IBS drugs), Viscera Labs, (USA 2021) (IBS-diarrhoea), Dr. Falk Pharma (2020) (EoE), Censa, Wellesley MA USA (2019) (Diabetic gastroparesis), Cadila PharmIncaceuticals (CME) (2019), Progenity Inc. San Diego, (USA 2019) (Intestinal capsule), Sanofi-aventis, Sydney (2019) (Probiotic), Glutagen (2020) (Celiac disease), ARENA Pharmaceuticals (2019) (Abdominal pain), IsoThrive (2021) (oesophageal microbiome), BluMaiden (2021), Rose Pharma (2021) and Intrinsic Medicine (2021) outside the submitted work. In addition, Dr. Talley has a patent Nepean Dyspepsia Index (NDI) 1998, Biomarkers of IBS licenced, a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licenced to Mayo/Talley, a patent Nestec European Patent licenced, and a patent Singapore Provisional Patent “Microbiota Modulation Of BDNF Tissue Repair Pathway” issued. Committees: Australian Medical Council (AMC) [Council Member]; Australian Telehealth Integration Programme; MBS Review Taskforce; NHMRC Principal Committee (Research Committee) Asia Pacific Association of Medical Journal Editors. Boards: GESA Board Member, Sax Institute, Committees of the Presidents of Medical Colleges. Community group: Advisory Board, IFFGD (International Foundation for Functional GI Disorders). Miscellaneous: Avant Foundation (judging of research grants). Editorial: Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, Med (Journal of Cell Press). Dr. Talley is supported by funding from the National Health and Medical Research Council (NHMRC) to the Centre for Research Excellence in Digestive Health and he holds an NHMRC Investigator grant.
SK: Grant/research support: National Health and Medical Research Council (Ideas Grant and Centre for Research Excellence) Viscera Labs (Research contract) and Microba Life Science (research contract). Consultant/advisory boards: Gossamer Bio (Scientific Advisory Board), Anatara Lifescience (Scientific Advisory Board), and Microba Life Science (Consultancy).
Figures

References
-
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150(6):1262–1279.e2. - PubMed
-
- Kibune Nagasako C, et al. Irritable bowel syndrome subtypes: clinical and psychological features, body mass index and comorbidities. Rev Esp Enferm Dig. 2016;108(2):59–64. - PubMed
-
- Burns G, et al. Evidence for local and systemic immune activation in functional dyspepsia and the irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2019;114(3):429–436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

