Antiplatelet therapy after percutaneous coronary intervention
- PMID: 35354550
- PMCID: PMC9896394
- DOI: 10.4244/EIJ-D-21-00904
Antiplatelet therapy after percutaneous coronary intervention
Abstract
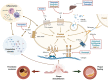
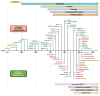
Antiplatelet therapy is key to reducing local thrombotic complications and systemic ischaemic events among patients undergoing percutaneous coronary interventions (PCI), but it is inevitably associated with increased bleeding. The continuous refinement in stent technologies, together with the high incidence of ischaemic recurrences after PCI and the understanding of prognostic implications associated with bleeding, have led to a substantial evolution in antiplatelet treatment regimens over the past decades. Numerous investigations have been conducted to better stratify patients undergoing PCI according to their ischaemic and bleeding risks and to implement antithrombotic regimens accordingly. Evidence from these investigations have resulted in a number of antithrombotic treatment options as recommended by recent guidelines. In this State-of-the-Art review we provide the rationale, summarise the evidence, and discuss current and future directions of antiplatelet treatment regimens after PCI.
Conflict of interest statement
D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical, outside the present work. D.J. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. J.P Collet has received financial support/sponsorship for research support, consultation or speaker fees from the following companies: AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Lead-Up, Medtronic, WebMD and Sanofi Aventis. M.L. O'Donoghue declares that she has received grants via Brigham and Women’s Hospital from Amgen, Novartis, AstraZeneca, Janssen, Intarcia, Merck, and Pfizer and honararia from Novartis, AstraZeneca, Amgen, and Janssen. The other authors have no conflicts of interest to declare.
Figures




Comment in
-
Temporal variations in ischemic and bleeding event risks after acute coronary syndrome during dual antiplatelet therapy.Int J Cardiol. 2023 Dec 1;392:131340. doi: 10.1016/j.ijcard.2023.131340. Epub 2023 Sep 9. Int J Cardiol. 2023. PMID: 37678433
References
-
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14(14):1435–534. - PubMed
-
- Cao D, Chandiramani R, Chiarito M, Claessen BE, Mehran R. Evolution of antithrombotic therapy in patients undergoing percutaneous coronary intervention: a 40-year journey. Eur Heart J. 2020;42:339–51. - PubMed
-
- Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA Versus ESC Guidelines on Dual Antiplatelet Therapy: JACC Guideline Comparison. J Am Coll Cardiol. 2018;72:2915–31. - PubMed
-
- Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med. 2011;364:453–64. - PubMed
-
- Moon JY, Franchi F, Rollini F, Angiolillo DJ. Evolution of Coronary Stent Technology and Implications for Duration of Dual Antiplatelet Therapy. Prog Cardiovasc Dis. 2018;60:478–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

