Long-Term Efficacy and Safety of Repeated Rituximab to Maintain Remission in Idiopathic Childhood Nephrotic Syndrome: An International Study
- PMID: 35354600
- PMCID: PMC9161790
- DOI: 10.1681/ASN.2021111472
Long-Term Efficacy and Safety of Repeated Rituximab to Maintain Remission in Idiopathic Childhood Nephrotic Syndrome: An International Study
Abstract
Background: Long-term outcomes after multiple courses of rituximab among children with frequently relapsing, steroid-dependent nephrotic syndrome (FRSDNS) are unknown.
Methods: A retrospective cohort study at 16 pediatric nephrology centers from ten countries in Asia, Europe, and North America included children with FRSDNS who received two or more courses of rituximab. Primary outcomes were relapse-free survival and adverse events.
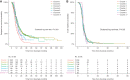
Results: A total of 346 children (age, 9.8 years; IQR, 6.6-13.5 years; 73% boys) received 1149 courses of rituximab. A total of 145, 83, 50, 28, 22, and 18 children received two, three, four, five, six, and seven or more courses, respectively. Median (IQR) follow-up was 5.9 (4.3-7.7) years. Relapse-free survival differed by treatment courses (clustered log-rank test P<0.001). Compared with the first course (10.0 months; 95% CI, 9.0 to 10.7 months), relapse-free period and relapse risk progressively improved after subsequent courses (12.0-16.0 months; HRadj, 0.03-0.13; 95% CI, 0.01 to 0.18; P<0.001). The duration of B-cell depletion remained similar with repeated treatments (6.1 months; 95% CI, 6.0 to 6.3 months). Adverse events were mostly mild; the most common adverse events were hypogammaglobulinemia (50.9%), infection (4.5%), and neutropenia (3.7%). Side effects did not increase with more treatment courses nor a higher cumulative dose. Only 78 of the 353 episodes of hypogammaglobulinemia were clinically significant. Younger age at presentation (2.8 versus 3.3 years; P=0.05), age at first rituximab treatment (8.0 versus 10.0 years; P=0.01), and history of steroid resistance (28% versus 18%; P=0.01) were associated with significant hypogammaglobulinemia. All 53 infective episodes resolved, except for one patient with hepatitis B infection and another with EBV infection. There were 42 episodes of neutropenia, associated with history of steroid resistance (30% versus 20%; P=0.04). Upon last follow-up, 332 children (96%) had normal kidney function.
Conclusions: Children receiving repeated courses of rituximab for FRSDNS experience an improving clinical response. Side effects appear acceptable, but significant complications can occur. These findings support repeated rituximab use in FRSDNS.
Keywords: biologics; children; hypogammaglobulinemia; nephrotic syndrome; neutropenia; rituximab.
Copyright © 2022 by the American Society of Nephrology.
Figures

References
-
- Webb NJA, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. ; PREDNOS Collaborative Group : Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: Phase III randomised controlled trial and economic evaluation. BMJ 365: l1800, 2019 - PMC - PubMed
-
- Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. ; Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group : Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384: 1273–1281, 2014 - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

