Efficacy and safety of an orally administered DGAT2 inhibitor alone or coadministered with a liver-targeted ACC inhibitor in adults with non-alcoholic steatohepatitis (NASH): rationale and design of the phase II, dose-ranging, dose-finding, randomised, placebo-controlled MIRNA (Metabolic Interventions to Resolve NASH with fibrosis) study
- PMID: 35354614
- PMCID: PMC8968568
- DOI: 10.1136/bmjopen-2021-056159
Efficacy and safety of an orally administered DGAT2 inhibitor alone or coadministered with a liver-targeted ACC inhibitor in adults with non-alcoholic steatohepatitis (NASH): rationale and design of the phase II, dose-ranging, dose-finding, randomised, placebo-controlled MIRNA (Metabolic Interventions to Resolve NASH with fibrosis) study
Abstract
Introduction: Small molecule inhibitors of the terminal step in intrahepatic triglyceride synthesis (diacylglycerol acyltransferase 2 inhibitor (DGAT2i, PF-06865571, ervogastat)) and upstream blockade of de novo lipogenesis via acetyl-coenzyme A carboxylase inhibitor (ACCi, PF-05221304, clesacostat) showed promise in reducing hepatic steatosis in early clinical trials. This study assesses efficacy and safety of these metabolic interventions to resolve non-alcoholic steatohepatitis (NASH) with fibrosis.
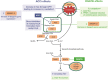
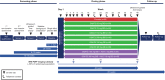
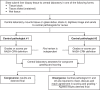
Methods and analysis: This phase II, randomised, dose-ranging, dose-finding study evaluates DGAT2i 25-300 mg two times per day (BID) or 150-300 mg once a day, DGAT2i 150-300 mg BID+ACCi 5-10 mg BID coadministration or matching placebo in a planned 450 adults with biopsy-confirmed NASH and liver fibrosis stages 2-3 from approximately 220 sites in 11 countries across North America, Europe and Asia. A triage approach including double-confirmation via non-invasive markers is included prior to screening/baseline liver biopsy. On confirmation of histological diagnosis, participants enter a ≥6-week run-in period, then a 48-week double-blind, double-dummy dosing period. The primary endpoint is the proportion of participants achieving histological NASH resolution without worsening fibrosis, ≥1 stage improvement in fibrosis without worsening NASH, or both, assessed by central pathologists. Other endpoints include assessment of hepatic steatosis (imaging substudy), overall safety and tolerability, and evaluation of blood-based biomarkers and quantitative ultrasound parameters over time.
Ethics and dissemination: Metabolic Interventions to Resolve NASH with fibrosis (MIRNA) is conducted in accordance with the Declaration of Helsinki and Council for International Organisations of Medical Sciences (CIOMS) International Ethical Guidelines, International Council on Harmonisation Good Clinical Practice guidelines, applicable laws and regulations, including privacy laws. Local independent review board/ethics committees (IRB/ECs) review/approve the protocol, any amendments, informed consent and other forms. Participants provide written informed consent. Details of all IRB/ECs, as well as results, will be published in a peer-reviewed journal and publicly disclosed through ClinicalTrials.gov, EudraCT, and/or www.pfizer.com and other public registries as per applicable local laws/regulations.
Trial registration number: NCT04321031.
Keywords: clinical trials; hepatology; histopathology; magnetic resonance imaging.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NBA, AD, DSL, MV and CY are employees of, and hold stock or stock options with, Pfizer Inc. QMA has received fees for consultancy on behalf of Newcastle University, grant funding via the EU IMI2 scheme and speaker fees from Pfizer Inc in relation to the submitted work. QMA has received grant funding from AbbVie, Allergan/Tobira, AstraZeneca, Genfit SA, GlaxoSmithKline, Glympse Bio, Intercept Pharma Europe Ltd (via the EU IMI2 scheme), Novartis Pharma AG, Pfizer Inc (via the EU IMI2 scheme); speaker fees from Bristol Myers Squibb, Gilead, Kenes, Novo Nordisk, Pfizer Inc; consultancy fees on behalf of Newcastle University from 89Bio, Allergan/Tobira, Altimmune, AstraZeneca, Axcella, BGMBio, Blade, BNN Cardio, Bristol Myers Squibb, Celgene, Cirius, CymaBay, E3Bio, EcoR1, Eli Lilly & Co, Galmed, Genentech, Genfit SA, Gilead, Grunthal, HistoIndex, Imperial Innovations, Indalo, Intercept Pharma Europe Ltd., Inventiva, IQVIA, Janssen, Madrigal, MedImmune, Metacrine, NewGene, NGMBio, North Sea Therapeutics, Novartis Pharma AG, Novo Nordisk, PathAI, Pfizer Inc, Poxel, Raptor Pharma, Servier, Terns, Viking Therapeutics, outside the submitted work. VW-SW has received medical writing and article processing charges support from Pfizer Inc for the submitted work. VW-SW’s institution has received grant funding from Gilead and support for meetings attendance from AbbVie and Gilead; VW-SW has received consulting fees and payments for participation on a Data Safety Monitoring Board or Advisory Board from 3V-Bio, AbbVie, Allergan, Boehringer Ingelheim, Centre for Outcomes Research in Liver Diseases, Echosens, Gilead, Hanmi Pharmaceutical, Intercept, Inventiva, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer Inc, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, Terns; VW-SW has received payment or honoraria for lectures, presentations, speakers bureaux, manuscript writing or educational events from Abbott, AbbVie, Bristol Myers Squibb, Echosens, Gilead; VW-SV has stock or stock options in Illuminatio Medical Technology Ltd. FT’s institution has received grants or contracts from Allergan, Bristol Myers Squibb, Galapagos, Gilead and Inventiva; FT has received consulting fees from AbbVie, Allergan, Boehringer Ingelheim, Bristol Myers Squibb, Galapagos, Gilead, Ionis, Ipsen, Inventiva, Novartis, Pfizer Inc and Roche; FT has received payment or honoraria for lectures, presentations, speakers bureaux, manuscript writing or educational events from Falk, Intercept and Gilead; he has received payment for expert testimony from Alnylam. NA has received grants or contracts from Akero, Allergan, Bristol Myers Squibb, DSM, Genentech, Genfit, Gilead, Intercept, Inventiva, Madrigal, NGMBio, North Sea Therapeutics, Novo Nordisk, Pfizer Inc, Poxel and Zydus; NA has received payment or honoraria for lectures, presentations, speakers bureaux, manuscript writing or educational events from Gilead and Intercept.MC and AN declare no competing interests. A patent rationalising the invention of administering DGAT2i+ACCi to mitigate effects of ACCi alone has been submitted.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
