Protein arginine N-methyltransferase 4 (PRMT4) contributes to lymphopenia in experimental sepsis
- PMID: 35354645
- PMCID: PMC9522923
- DOI: 10.1136/thoraxjnl-2021-217526
Protein arginine N-methyltransferase 4 (PRMT4) contributes to lymphopenia in experimental sepsis
Abstract
Background: One hallmark of sepsis is the reduced number of lymphocytes, termed lymphopenia, that occurs from decreased lymphocyte proliferation or increased cell death contributing to immune suppression. Histone modification enzymes regulate immunity by their epigenetic and non-epigenetic functions; however, the role of these enzymes in lymphopenia remains elusive.
Methods: We used molecular biological approaches to investigate the high expression and function of a chromatin modulator protein arginine N-methyltransferase 4 (PRMT4)/coactivator-associated arginine methyltransferase 1 in human samples from septic patients and cellular and animal septic models.
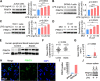
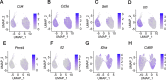
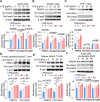
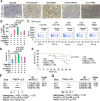
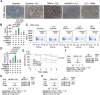
Results: We identified that PRMT4 is elevated systemically in septic patients and experimental sepsis. Gram-negative bacteria and their derived endotoxin lipopolysaccharide (LPS) increased PRMT4 in B and T lymphocytes and THP-1 monocytes. Single-cell RNA sequencing results indicate an increase of PRMT4 gene expression in activated T lymphocytes. Augmented PRMT4 is crucial for inducing lymphocyte apoptosis but not monocyte THP-1 cells. Ectopic expression of PRMT4 protein caused substantial lymphocyte death via caspase 3-mediated cell death signalling, and knockout of PRMT4 abolished LPS-mediated lymphocyte death. PRMT4 inhibition with a small molecule compound attenuated lymphocyte death in complementary models of sepsis.
Conclusions: These findings demonstrate a previously uncharacterised role of a key chromatin modulator in lymphocyte survival that may shed light on devising therapeutic modalities to lessen the severity of septic immunosuppression.
Keywords: ARDS; Bacterial Infection; Lymphocyte Biology; Pneumonia; Respiratory Infection.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL142997/HL/NHLBI NIH HHS/United States
- R01 HL097376/HL/NHLBI NIH HHS/United States
- K24 HL143285/HL/NHLBI NIH HHS/United States
- R01 HL136143/HL/NHLBI NIH HHS/United States
- R01 HL125435/HL/NHLBI NIH HHS/United States
- R01 HL142084/HL/NHLBI NIH HHS/United States
- R01 HL149719/HL/NHLBI NIH HHS/United States
- R01 HL096376/HL/NHLBI NIH HHS/United States
- R01 HL081784/HL/NHLBI NIH HHS/United States
- R01 HL098174/HL/NHLBI NIH HHS/United States
- I01 CX001048/CX/CSRD VA/United States
- K23 HL139987/HL/NHLBI NIH HHS/United States
- I01 CX000105/CX/CSRD VA/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
