Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline
- PMID: 35354777
- PMCID: PMC10259184
- DOI: 10.14309/ajg.0000000000001680
Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline
Abstract
Barrett's esophagus (BE) is a common condition associated with chronic gastroesophageal reflux disease. BE is the only known precursor to esophageal adenocarcinoma, a highly lethal cancer with an increasing incidence over the last 5 decades. These revised guidelines implement Grading of Recommendations, Assessment, Development, and Evaluation methodology to propose recommendations for the definition and diagnosis of BE, screening for BE and esophageal adenocarcinoma, surveillance of patients with known BE, and the medical and endoscopic treatment of BE and its associated early neoplasia. Important changes since the previous iteration of this guideline include a broadening of acceptable screening modalities for BE to include nonendoscopic methods, liberalized intervals for surveillance of short-segment BE, and volume criteria for endoscopic therapy centers for BE. We recommend endoscopic eradication therapy for patients with BE and high-grade dysplasia and those with BE and low-grade dysplasia. We propose structured surveillance intervals for patients with dysplastic BE after successful ablation based on the baseline degree of dysplasia. We could not make recommendations regarding chemoprevention or use of biomarkers in routine practice due to insufficient data.
Copyright © 2022 by The American College of Gastroenterology.
Conflict of interest statement
Figures




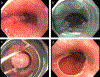
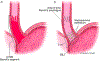
Comment in
-
Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline.Am J Gastroenterol. 2022 Nov 1;117(11):1880. doi: 10.14309/ajg.0000000000001896. Am J Gastroenterol. 2022. PMID: 35973173 No abstract available.
References
-
- Barrett NR. Chronic peptic ulcer of the oesophagus and “oesophagitis.” Br J Surg 1950;38:175–82. - PubMed
-
- Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24. - PubMed
-
- Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest Endosc 2019;90:707–17. - PubMed
-
- Thrift AP, El-Serag HB, Kanwal F. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;14(2):122–32. - PubMed

