Cyclophilin anaCyp40 regulates photosystem assembly and phycobilisome association in a cyanobacterium
- PMID: 35354803
- PMCID: PMC8967839
- DOI: 10.1038/s41467-022-29211-w
Cyclophilin anaCyp40 regulates photosystem assembly and phycobilisome association in a cyanobacterium
Abstract
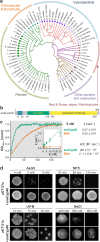
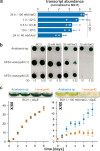
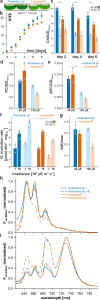
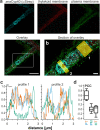
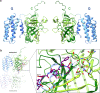
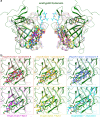
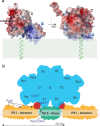
Cyclophilins, or immunophilins, are proteins found in many organisms including bacteria, plants and humans. Most of them display peptidyl-prolyl cis-trans isomerase activity, and play roles as chaperones or in signal transduction. Here, we show that cyclophilin anaCyp40 from the cyanobacterium Anabaena sp. PCC 7120 is enzymatically active, and seems to be involved in general stress responses and in assembly of photosynthetic complexes. The protein is associated with the thylakoid membrane and interacts with phycobilisome and photosystem components. Knockdown of anacyp40 leads to growth defects under high-salt and high-light conditions, and reduced energy transfer from phycobilisomes to photosystems. Elucidation of the anaCyp40 crystal structure at 1.2-Å resolution reveals an N-terminal helical domain with similarity to PsbQ components of plant photosystem II, and a C-terminal cyclophilin domain with a substrate-binding site. The anaCyp40 structure is distinct from that of other multi-domain cyclophilins (such as Arabidopsis thaliana Cyp38), and presents features that are absent in single-domain cyclophilins.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Andrzej G. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity—targets—functions. Curr. Top. Med. Chem. 2003;3:1315–1347. - PubMed
-
- Schiene-Fischer C. Multidomain peptidyl prolyl cis/trans isomerases. Biochim. Biophys. Acta. 2015;1850:2005–2016. - PubMed
-
- Romano P, Gray J, Horton P, Luan S. Plant immunophilins: functional versatility beyond protein maturation. N. Phytol. 2005;166:753–769. - PubMed
-
- Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. - PubMed
-
- Stewart DE, Sarkar A, Wampler JE. Occurrence and role of cis peptide bonds in protein structures. J. Mol. Biol. 1990;214:253–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

