Neutrophils direct preexisting matrix to initiate repair in damaged tissues
- PMID: 35354953
- PMCID: PMC8986538
- DOI: 10.1038/s41590-022-01166-6
Neutrophils direct preexisting matrix to initiate repair in damaged tissues
Abstract
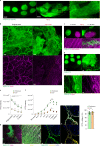
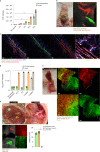
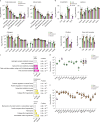
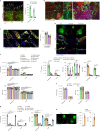
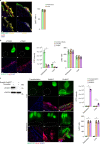
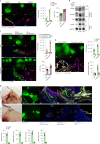
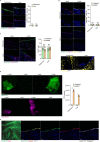
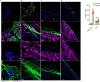
Internal organs heal injuries with new connective tissue, but the cellular and molecular events of this process remain obscure. By tagging extracellular matrix around the mesothelium lining in mouse peritoneum, liver and cecum, here we show that preexisting matrix was transferred across organs into wounds in various injury models. Using proteomics, genetic lineage-tracing and selective injury in juxtaposed organs, we found that the tissue of origin for the transferred matrix likely dictated the scarring or regeneration of the healing tissue. Single-cell RNA sequencing and genetic and chemical screens indicated that the preexisting matrix was transferred by neutrophils dependent on the HSF-integrin AM/B2-kindlin3 cascade. Pharmacologic inhibition of this axis prevented matrix transfer and the formation of peritoneal adhesions. Matrix transfer was thus an early event of wound repair and provides a therapeutic window to dampen scaring across a range of conditions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Neutrophils guide pre-existing matrix into injured organs to initiate tissue repair.Nat Immunol. 2022 Apr;23(4):472-473. doi: 10.1038/s41590-022-01173-7. Nat Immunol. 2022. PMID: 35354952 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

