Heterogeneity of type 2 innate lymphoid cells
- PMID: 35354980
- PMCID: PMC8966870
- DOI: 10.1038/s41577-022-00704-5
Heterogeneity of type 2 innate lymphoid cells
Abstract
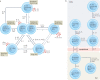
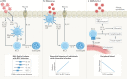
More than a decade ago, type 2 innate lymphoid cells (ILC2s) were discovered to be members of a family of innate immune cells consisting of five subsets that form a first line of defence against infections before the recruitment of adaptive immune cells. Initially, ILC2s were implicated in the early immune response to parasitic infections, but it is now clear that ILC2s are highly diverse and have crucial roles in the regulation of tissue homeostasis and repair. ILC2s can also regulate the functions of other type 2 immune cells, including T helper 2 cells, type 2 macrophages and eosinophils. Dysregulation of ILC2s contributes to type 2-mediated pathology in a wide variety of diseases, potentially making ILC2s attractive targets for therapeutic interventions. In this Review, we focus on the spectrum of ILC2 phenotypes that have been described across different tissues and disease states with an emphasis on human ILC2s. We discuss recent insights in ILC2 biology and suggest how this knowledge might be used for novel disease treatments and improved human health.
© 2022. Springer Nature Limited.
Conflict of interest statement
H.S. is a consultant for GlaxoSmithKline. J.M. declares no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

