Human distal airways contain a multipotent secretory cell that can regenerate alveoli
- PMID: 35355013
- PMCID: PMC9297319
- DOI: 10.1038/s41586-022-04552-0
Human distal airways contain a multipotent secretory cell that can regenerate alveoli
Abstract
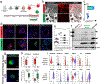
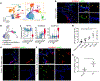
The human lung differs substantially from its mouse counterpart, resulting in a distinct distal airway architecture affected by disease pathology in chronic obstructive pulmonary disease. In humans, the distal branches of the airway interweave with the alveolar gas-exchange niche, forming an anatomical structure known as the respiratory bronchioles. Owing to the lack of a counterpart in mouse, the cellular and molecular mechanisms that govern respiratory bronchioles in the human lung remain uncharacterized. Here we show that human respiratory bronchioles contain a unique secretory cell population that is distinct from cells in larger proximal airways. Organoid modelling reveals that these respiratory airway secretory (RAS) cells act as unidirectional progenitors for alveolar type 2 cells, which are essential for maintaining and regenerating the alveolar niche. RAS cell lineage differentiation into alveolar type 2 cells is regulated by Notch and Wnt signalling. In chronic obstructive pulmonary disease, RAS cells are altered transcriptionally, corresponding to abnormal alveolar type 2 cell states, which are associated with smoking exposure in both humans and ferrets. These data identify a distinct progenitor in a region of the human lung that is not found in mouse that has a critical role in maintaining the gas-exchange compartment and is altered in chronic lung disease.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures














Comment in
-
Secretory RAS cells: A novel AT2 progenitor in the human transitional bronchioles.Allergy. 2022 Sep;77(9):2866-2868. doi: 10.1111/all.15402. Epub 2022 Jun 13. Allergy. 2022. PMID: 35665940 No abstract available.
References
-
- Weibel ER, Sapoval B & Filoche M Design of peripheral airways for efficient gas exchange. Respir. Physiol. Neurobiol 148, 3–21 (2005). - PubMed
MeSH terms
Grants and funding
- R03 HL135227/HL/NHLBI NIH HHS/United States
- R01 HL087825/HL/NHLBI NIH HHS/United States
- P30 DK072482/DK/NIDDK NIH HHS/United States
- T32 HL007586/HL/NHLBI NIH HHS/United States
- U01 HL134745/HL/NHLBI NIH HHS/United States
- K08 HL163398/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- R37 DK047967/DK/NIDDK NIH HHS/United States
- U01 HL134766/HL/NHLBI NIH HHS/United States
- R01 HL165404/HL/NHLBI NIH HHS/United States
- K23 HL121406/HL/NHLBI NIH HHS/United States
- P01 HL152960/HL/NHLBI NIH HHS/United States
- K08 HL150226/HL/NHLBI NIH HHS/United States
- K99 HL155785/HL/NHLBI NIH HHS/United States
- R35 HL135816/HL/NHLBI NIH HHS/United States
- U01 HL148857/HL/NHLBI NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- HL148857/NH/NIH HHS/United States
- F32 HL143931/HL/NHLBI NIH HHS/United States
- R01 DK047967/DK/NIDDK NIH HHS/United States
- HL134745/NH/NIH HHS/United States
- R35 HL150767/HL/NHLBI NIH HHS/United States
- K08 HL130586/HL/NHLBI NIH HHS/United States
- U01 HL152978/HL/NHLBI NIH HHS/United States
- R01 HL132999/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

