Obesity alters pathology and treatment response in inflammatory disease
- PMID: 35355021
- PMCID: PMC9165753
- DOI: 10.1038/s41586-022-04536-0
Obesity alters pathology and treatment response in inflammatory disease
Abstract
Decades of work have elucidated cytokine signalling and transcriptional pathways that control T cell differentiation and have led the way to targeted biologic therapies that are effective in a range of autoimmune, allergic and inflammatory diseases. Recent evidence indicates that obesity and metabolic disease can also influence the immune system1-7, although the mechanisms and effects on immunotherapy outcomes remain largely unknown. Here, using two models of atopic dermatitis, we show that lean and obese mice mount markedly different immune responses. Obesity converted the classical type 2 T helper (TH2)-predominant disease associated with atopic dermatitis to a more severe disease with prominent TH17 inflammation. We also observed divergent responses to biologic therapies targeting TH2 cytokines, which robustly protected lean mice but exacerbated disease in obese mice. Single-cell RNA sequencing coupled with genome-wide binding analyses revealed decreased activity of nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) in TH2 cells from obese mice relative to lean mice. Conditional ablation of PPARγ in T cells revealed that PPARγ is required to focus the in vivo TH response towards a TH2-predominant state and prevent aberrant non-TH2 inflammation. Treatment of obese mice with a small-molecule PPARγ agonist limited development of TH17 pathology and unlocked therapeutic responsiveness to targeted anti-TH2 biologic therapies. These studies reveal the effects of obesity on immunological disease and suggest a precision medicine approach to target the immune dysregulation caused by obesity.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Figures









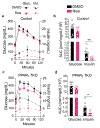
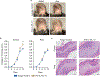



Comment in
-
Obesity amplifies TH17-type pathology in atopic diseases.Nat Rev Immunol. 2022 May;22(5):274-275. doi: 10.1038/s41577-022-00721-4. Nat Rev Immunol. 2022. PMID: 35393549 No abstract available.
References
-
- Michelet X et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol 19, 1330–1640 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- 16BGIA27790079/AHA/American Heart Association-American Stroke Association/United States
- K38 HL154202/HL/NHLBI NIH HHS/United States
- P01 HL107202/HL/NHLBI NIH HHS/United States
- R01 DK120480/DK/NIDDK NIH HHS/United States
- P30 GM127211/GM/NIGMS NIH HHS/United States
- U01 AI52038/NH/NIH HHS/United States
- S10 OD023689/OD/NIH HHS/United States
- R01 HL080414/HL/NHLBI NIH HHS/United States
- R01 AI107027/AI/NIAID NIH HHS/United States
- R37 AI052453/AI/NIAID NIH HHS/United States
- R01 HL105278/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R38 HL143581/HL/NHLBI NIH HHS/United States
- IK2 BX001313/BX/BLRD VA/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- R01 DK121760/NH/NIH HHS/United States
- R01 AR076082/AR/NIAMS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 HL109102/HL/NHLBI NIH HHS/United States
- R21 AI154919/AI/NIAID NIH HHS/United States
- F30 DK096828/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 AI151123/AI/NIAID NIH HHS/United States
- P01 HL147835/HL/NHLBI NIH HHS/United States
- R01 AI153185/AI/NIAID NIH HHS/United States
- T32 GM007198/GM/NIGMS NIH HHS/United States
- P20 GM103527/GM/NIGMS NIH HHS/United States
- R01 DK057978/DK/NIDDK NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States

