Alzheimer-like tau accumulation in dentate gyrus mossy cells induces spatial cognitive deficits by disrupting multiple memory-related signaling and inhibiting local neural circuit
- PMID: 35355405
- PMCID: PMC9124302
- DOI: 10.1111/acel.13600
Alzheimer-like tau accumulation in dentate gyrus mossy cells induces spatial cognitive deficits by disrupting multiple memory-related signaling and inhibiting local neural circuit
Abstract
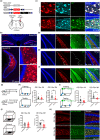
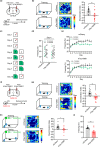
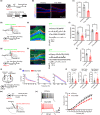
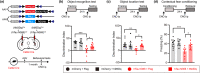
Abnormal tau accumulation and spatial memory loss constitute characteristic pathology and symptoms of Alzheimer disease (AD). Yet, the intrinsic connections and the mechanism between them are not fully understood. In the current study, we observed a prominent accumulation of the AD-like hyperphosphorylated and truncated tau (hTau N368) proteins in hippocampal dentate gyrus (DG) mossy cells of 3xTg-AD mice. Further investigation demonstrated that the ventral DG (vDG) mossy cell-specific overexpressing hTau for 3 months induced spatial cognitive deficits, while expressing hTau N368 for only 1 month caused remarkable spatial cognitive impairment with more prominent tau pathologies. By in vivo electrophysiological and optic fiber recording, we observed that the vDG mossy cell-specific overexpression of hTau N368 disrupted theta oscillations with local neural network inactivation in the dorsal DG subset, suggesting impairment of the ventral to dorsal neural circuit. The mossy cell-specific transcriptomic data revealed that multiple AD-associated signaling pathways were disrupted by hTau N368, including reduction of synapse-associated proteins, inhibition of AKT and activation of glycogen synthase kinase-3β. Importantly, chemogenetic activating mossy cells efficiently attenuated the hTau N368-induced spatial cognitive deficits. Together, our findings indicate that the mossy cell pathological tau accumulation could induce the AD-like spatial memory deficit by inhibiting the local neural network activity, which not only reveals new pathogenesis underlying the mossy cell-related spatial memory loss but also provides a mouse model of Mossy cell-specific hTau accumulation for drug development in AD and the related tauopathies.
Keywords: Alzheimer's disease; hTau N368; hippocampus; mossy cell; spatial memory.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Botterill, J. J. , Lu, Y.‐L. , LaFrancois, J. J. , Bernstein, H. L. , Alcantara‐Gonzalez, D. , Jain, S. , Leary, P. , & Scharfman, H. E. (2019). An excitatory and epileptogenic effect of dentate gyrus mossy cells in a mouse model of epilepsy. Cell Reports, 29(9), 2875–2889.e2876. 10.1016/j.celrep.2019.10.100 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

