Neurotoxicity: A Rare Side Effect of Programmed Cell Death 1 (PD-1) Inhibitors
- PMID: 35355539
- PMCID: PMC8957660
- DOI: 10.7759/cureus.22584
Neurotoxicity: A Rare Side Effect of Programmed Cell Death 1 (PD-1) Inhibitors
Abstract
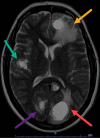
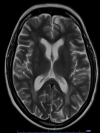
Immunotherapy is a biological therapy that helps the body's immune system to fight against cancer cells. The Food and Drug Administration (FDA) approved the first immune checkpoint inhibitor in 2011. Since 2011, many immune checkpoint inhibitors have been approved. Programmed cell death 1 (PD-1) inhibitors are now commonly used in multiple malignancies due to their remarkable response. Thus, immune-related adverse events are now coming into the limelight due to the increasing use of PD-1 inhibitors. Here, we present a case of a 54-year-old female with non-small cell lung cancers (NSCLC) treated with pembrolizumab and later presented with severe neurotoxicity.
Keywords: anti-pd-1; immuno-checkpoint inhibitor; immunotherapy adverse effect; lung cancer; non-small cell lung cancer (nsclc).
Copyright © 2022, Ehsanullah et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. Feng S, Coward J, McCaffrey E, Coucher J, Kalokerinos P, O'Byrne K. J Thorac Oncol. 2017;12:1626–1635. - PubMed
-
- Therapeutic prospects of targeting myeloid-derived suppressor cells and immune checkpoints in cancer. Toor SM, Elkord E. Immunol Cell Biol. 2018;96:888–897. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
