Identification of MAGEC2/CT10 as a High Calcium-Inducible Gene in Triple-Negative Breast Cancer
- PMID: 35355564
- PMCID: PMC8959981
- DOI: 10.3389/fendo.2022.816598
Identification of MAGEC2/CT10 as a High Calcium-Inducible Gene in Triple-Negative Breast Cancer
Abstract
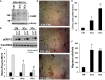
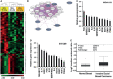
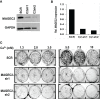
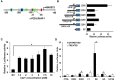
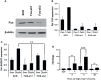
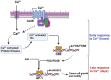
The expression of the melanoma/cancer-testis antigen MAGEC2/CT10 is restricted to germline cells, but like most cancer-testis antigens, it is frequently upregulated in advanced breast tumors and other malignant tumors. However, the physiological cues that trigger the expression of this gene during malignancy remain unknown. Given that malignant breast cancer is often associated with skeletal metastasis and co-morbidities such as cancer-induced hypercalcemia, we evaluated the effect of high Ca2+ on the calcium-sensing receptor (CaSR) and potential mechanisms underlying the survival of triple-negative breast cancer (TNBC) cells at high Ca2+. We show that chronic exposure of TNBC cells to high Ca2+ decreased the sensitivity of CaSR to Ca2+ but stimulated tumor cell growth and migration. Furthermore, high extracellular Ca2+ also stimulated the expression of early response genes such as FOS/FOSB and a unique set of genes associated with malignant tumors, including MAGEC2. We further show that the MAGEC2 proximal promoter is Ca2+ inducible and that FOS/FOSB binds to this promoter in a Ca2+- dependent manner. Finally, downregulation of MAGEC2 strongly inhibited the growth of TNBC cells in vitro. These data suggest for the first time that MAGEC2 is a high Ca2+ inducible gene and that aberrant expression of MAGEC2 in malignant TNBC tissues is at least in part mediated by an increase in circulating Ca2+via the AP-1 transcription factor.
Keywords: AP-1; MAGEC2; TNBC; breast cancer; calcium signaling; calcium-sensing receptor; cell motility; cell proliferation.
Copyright © 2022 Beasley, Widatalla, Whalen, Williams, Korolkova, Namba, Pratap, Ochieng and Sakwe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

