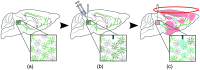
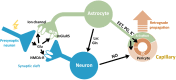
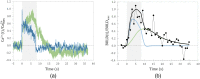
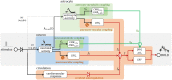
Hybrid fiber optic-fMRI for multimodal cell-specific recording and manipulation of neural activity in rodents
- PMID: 35355657
- PMCID: PMC8936941
- DOI: 10.1117/1.NPh.9.3.032206
Hybrid fiber optic-fMRI for multimodal cell-specific recording and manipulation of neural activity in rodents
Abstract
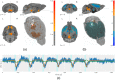
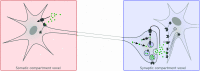
Significance: Multiscale imaging holds particular relevance to neuroscience, where it helps integrate the cellular and molecular biological scale, which is most accessible to interventions, with holistic organ-level evaluations, most relevant with respect to function. Being inextricably interdisciplinary, multiscale imaging benefits substantially from incremental technology adoption, and a detailed overview of the state-of-the-art is vital to an informed application of imaging methods. Aim: In this article, we lay out the background and methodological aspects of multimodal approaches combining functional magnetic resonance imaging (fMRI) with simultaneous optical measurement or stimulation. Approach: We focus on optical techniques as these allow, in conjunction with genetically encoded proteins (e.g. calcium indicators or optical signal transducers), unprecedented read-out and control specificity for individual cell-types during fMRI experiments, while leveraging non-interfering modalities. Results: A variety of different solutions for optical/fMRI methods has been reported ranging from bulk fluorescence recordings via fiber photometry to high resolution microscopy. In particular, the plethora of optogenetic tools has enabled the transformation of stimulus-evoked fMRI into a cell biological interrogation method. We discuss the capabilities and limitations of these genetically encoded molecular tools in the study of brain phenomena of great methodological and neuropsychiatric interest-such as neurovascular coupling (NVC) and neuronal network mapping. We provide a methodological description of this interdisciplinary field of study, and focus in particular on the limitations of the widely used blood oxygen level dependent (BOLD) signal and how multimodal readouts can shed light on the contributions arising from neurons, astrocytes, or the vasculature. Conclusion: We conclude that information from multiple signaling pathways must be incorporated in future forward models of the BOLD response to prevent erroneous conclusions when using fMRI as a surrogate measure for neural activity. Further, we highlight the potential of direct neuronal stimulation via genetically defined brain networks towards advancing neurophysiological understanding and better estimating effective connectivity.
Keywords: functional magnetic resonance imaging; multimodal; neuroimaging; neuroscience; optics; optogenetics; technology.
© 2022 The Authors.
Figures