HIF1α/VEGF Feedback Loop Contributes to 5-Fluorouracil Resistance
- PMID: 35355718
- PMCID: PMC8959760
- DOI: 10.3389/fphar.2022.851401
HIF1α/VEGF Feedback Loop Contributes to 5-Fluorouracil Resistance
Abstract
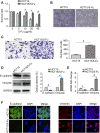
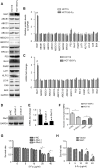
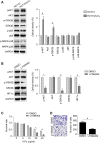
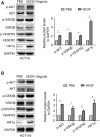
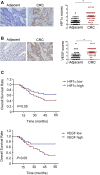
5-Fluorouracil (5-Fu) is one of the basic drugs in colorectal cancer (CRC) chemotherapy, and its efficacy is mainly limited by the acquisition of drug resistance. However, the underlying mechanisms remain unclear. In this study, hypoxia inducible factor 1α (HIF1α) was screened for high expression in 5-Fu resistant HCT115 cells, which displayed epithelial-mesenchymal transition (EMT) phenotype. Suppression of HIF1α reversed EMT phenotype, reduced glucose transporter 1 (Glut1) expression, a key molecule mediated drug resistance. Moreover, we unveiled that vascular endothelial growth factor (VEGF) was regulated by HIF1α and mediated HIF1α-maintained malignant phenotype of 5-Fu resistant cells. Further studies verified that AKT/GSK3β signaling was activated in resistant cells and controlled HIF1α expression. Interestingly, we demonstrated that VEGF could feedback up-regulate HIF1α via AKT/GSK3β signaling. Clinically, HIF1α and VEGF were high expressed and associated with survival and prognosis in CRC patients. In conclusion, our findings proposed that HIF1α/VEGF feedback loop contributed to 5-Fu resistance, which might be potential therapeutic targets.
Keywords: Akt/GSK3β; EMT; GLUT1; HIF1α; VEGF; drug resistance.
Copyright © 2022 Shi, Xu, Xiang, Li, Fan and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Factor inhibiting HIF1α (FIH-1) functions as a tumor suppressor in human colorectal cancer by repressing HIF1α pathway.Cancer Biol Ther. 2015;16(2):244-52. doi: 10.1080/15384047.2014.1002346. Cancer Biol Ther. 2015. PMID: 25602156 Free PMC article.
-
Jiedu Sangen decoction inhibits chemoresistance to 5-fluorouracil of colorectal cancer cells by suppressing glycolysis via PI3K/AKT/HIF-1α signaling pathway.Chin J Nat Med. 2021 Feb;19(2):143-152. doi: 10.1016/S1875-5364(21)60015-8. Chin J Nat Med. 2021. PMID: 33641785
-
ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer.J Exp Clin Cancer Res. 2022 Jan 8;41(1):15. doi: 10.1186/s13046-021-02229-6. J Exp Clin Cancer Res. 2022. PMID: 34998404 Free PMC article.
-
Drug resistance and cancer stem cells: the shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α.Clin Exp Pharmacol Physiol. 2017 Feb;44(2):153-161. doi: 10.1111/1440-1681.12693. Clin Exp Pharmacol Physiol. 2017. PMID: 27809360 Review.
-
Hypoxia inducible factor-1α: Its role in colorectal carcinogenesis and metastasis.Cancer Lett. 2015 Sep 28;366(1):11-8. doi: 10.1016/j.canlet.2015.06.005. Epub 2015 Jun 24. Cancer Lett. 2015. PMID: 26116902 Review.
Cited by
-
ECM1 regulates the resistance of colorectal cancer to 5-FU treatment by modulating apoptotic cell death and epithelial-mesenchymal transition induction.Front Pharmacol. 2022 Nov 2;13:1005915. doi: 10.3389/fphar.2022.1005915. eCollection 2022. Front Pharmacol. 2022. PMID: 36408224 Free PMC article.
-
Dichotomic Role of Low-Concentration EGCG in the Oxaliplatin Sensitivity of Colorectal Cancer Cells.Dokl Biochem Biophys. 2024 Apr;515(1):29-35. doi: 10.1134/S160767292360029X. Epub 2024 Jan 8. Dokl Biochem Biophys. 2024. PMID: 38189882 Free PMC article.
-
TRIM23 promotes 5-Fluorouracil resistance in colorectal cancer by upregulating GALNT4 expression.Apoptosis. 2025 Apr;30(3-4):751-767. doi: 10.1007/s10495-024-02060-2. Epub 2024 Dec 25. Apoptosis. 2025. PMID: 39720975 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

