Biological and Methotrexate Survival after Pregnancy in Patients With a Rheumatic Disease
- PMID: 35355725
- PMCID: PMC8959570
- DOI: 10.3389/fphar.2022.826034
Biological and Methotrexate Survival after Pregnancy in Patients With a Rheumatic Disease
Abstract
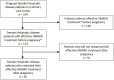
Objective: Patients with a rheumatic disease who discontinue their disease-modifying anti-rheumatic drug (DMARD) due to pregnancy often wonder if treatment will be as effective after pregnancy. This study investigates the effect of a temporary discontinuation of DMARDs due to pregnancy on the effectiveness of the same DMARD postpartum in patients with a rheumatic disease. Methods: Pregnant, rheumatic patients were derived from the Preconceptional Counseling in Active Rheumatoid Arthritis (PreCARA) cohort. DMARD-survival after pregnancy, for biological and methotrexate (MTX) therapy, was analyzed and compared to controls with stable DMARD-treatment from a retrospective cohort. Results: In total, 234 patients were included, of whom 114 patients had stable biological or MTX treatment before their pregnancy. After pregnancy, 40 out of 56 (71%) patients restarted their biological, for MTX this was 49%. One year after restart, and censoring for a following pregnancy, 88.9% of patients were still using their biological, and 85% still used their MTX (p = 0.92). Compared to the matched controls the survival after pregnancy was significantly lower 1 year after restart for both biologicals (98.3%) and MTX (99.6%); p = 0.002 and p < 0.001 respectively; 3 years after restart this significant difference was no longer observed (p = 0.50 and p = 0.33, respectively). Conclusion: Effective DMARD (biological or MTX) treatment before pregnancy that was discontinued due to pregnancy seems effective after pregnancy. Although DMARD-survival was higher in the control group 1 year after restart, the percentage of patients with effective treatment was still very good (>85%). In addition, this difference was no longer observed after 3 years.
Keywords: DMARD (disease modifying anti-rheumatic drug); biological; biological survival; pregnancy; rheumatic dieases.
Copyright © 2022 Tahmasian, Smeele, de Jong, Dolhain and van Mulligen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

