The Role of Aeromonas-Goblet Cell Interactions in Melatonin-Mediated Improvements in Sleep Deprivation-Induced Colitis
- PMID: 35355860
- PMCID: PMC8958064
- DOI: 10.1155/2022/8133310
The Role of Aeromonas-Goblet Cell Interactions in Melatonin-Mediated Improvements in Sleep Deprivation-Induced Colitis
Abstract
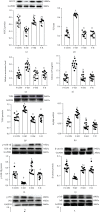
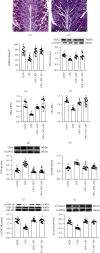
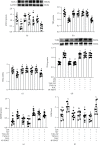
Background: Our previous studies demonstrated that melatonin could effectively ameliorate sleep deprivation- (SD-) caused oxidative stress-mediated gut microbiota disorder and colitis. The research further clarified the mechanism of melatonin in improving colitis from the perspective of the interaction between Aeromonas and goblet cells.
Methods: A seventy-two hours SD mouse model with or without melatonin intervention and fecal microbiota transplantation (FMT) to explore the vital position of Aeromonas-goblet cell interactions in melatonin improving SD-induced colitis. Moreover, Aeromonas or LPS-supplied mice were assessed, and the influence of melatonin on Aeromonas-goblet cell interactions-mediated oxidative stress caused colitis. Furthermore, in vitro experiment investigated the regulation mechanism of melatonin.
Results: Our study showed that SD induced colitis, with upregulation of Aeromonas and LPS levels and reductions in goblet cells number and MUC2 protein. Similarly, FMT from SD mice, Aeromonas veronii colonization, and LPS treatment restored the SD-like goblet cells number and MUC2 protein decrease and colitis. Moreover, LPS treatment downregulated the colonic antioxidant capacity. Yet, melatonin intervention reversed all consequence in SD, A.veronii colonization, and LPS-treated mice. In vitro, melatonin reversed A. veronii- or LPS-induced MUC2 depletion in mucus-secreting human HT-29 cells via increasing the expression level of Villin, Tff3, p-GSK-3β, β-catenin, and melatonin receptor 2 (MT2) and decreasing the level of p-IκB, p-P65, ROS, TLR4, and MyD88 proteins, while the improvement effect was blocked with pretreatment with a MT2 antagonist but were mimicked by TLR4 and GSK-3β antagonists and ROS scavengers.
Conclusions: Our results demonstrated that melatonin-mediated MT2 inhibits Aeromonas-goblet cell interactions to restore the level of MUC2 production via LPS/TLR4/MyD88/GSK-3β/ROS/NF-κB loop, further improving colitis in SD mice.
Copyright © 2022 Ting Gao et al.
Conflict of interest statement
The authors declare that they have no potential conflicts of interest, including any financial, personal, or other relationships, with other people or organizations.
Figures





Similar articles
-
Melatonin-Mediated Colonic Microbiota Metabolite Butyrate Prevents Acute Sleep Deprivation-Induced Colitis in Mice.Int J Mol Sci. 2021 Nov 2;22(21):11894. doi: 10.3390/ijms222111894. Int J Mol Sci. 2021. PMID: 34769321 Free PMC article.
-
Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation.Microbiome. 2023 Jan 31;11(1):17. doi: 10.1186/s40168-022-01452-3. Microbiome. 2023. PMID: 36721179 Free PMC article.
-
Melatonin Ameliorates Corticosterone-Mediated Oxidative Stress-Induced Colitis in Sleep-Deprived Mice Involving Gut Microbiota.Oxid Med Cell Longev. 2021 Jun 23;2021:9981480. doi: 10.1155/2021/9981480. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34257825 Free PMC article.
-
Melatonin-mediated intestinal microbiota homeostasis improves skin barrier damage involvement of gut-skin axis dysfunction in aging mice.Cell Signal. 2025 Sep;133:111859. doi: 10.1016/j.cellsig.2025.111859. Epub 2025 May 9. Cell Signal. 2025. PMID: 40349812
-
Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice.J Pineal Res. 2019 Aug;67(1):e12574. doi: 10.1111/jpi.12574. Epub 2019 Apr 12. J Pineal Res. 2019. PMID: 30929267
Cited by
-
Melatonin-Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions.Antioxidants (Basel). 2022 Nov 14;11(11):2244. doi: 10.3390/antiox11112244. Antioxidants (Basel). 2022. PMID: 36421432 Free PMC article. Review.
-
Mechanism of Action of Melatonin as a Potential Adjuvant Therapy in Inflammatory Bowel Disease and Colorectal Cancer.Nutrients. 2024 Apr 21;16(8):1236. doi: 10.3390/nu16081236. Nutrients. 2024. PMID: 38674926 Free PMC article. Review.
-
Helicobacter pylori promotes gastric intestinal metaplasia through activation of IRF3-mediated kynurenine pathway.Cell Commun Signal. 2023 Jun 16;21(1):141. doi: 10.1186/s12964-023-01162-9. Cell Commun Signal. 2023. PMID: 37328804 Free PMC article.
-
Caffeic Acid Alleviates Chronic Sleep Deprivation-Induced Intestinal Damage by Inhibiting the IMD Pathway in Drosophila.J Inflamm Res. 2025 Mar 10;18:3485-3498. doi: 10.2147/JIR.S500892. eCollection 2025. J Inflamm Res. 2025. PMID: 40093942 Free PMC article.
-
Melatonin as a Mediator of the Gut Microbiota-Host Interaction: Implications for Health and Disease.Antioxidants (Basel). 2023 Dec 23;13(1):34. doi: 10.3390/antiox13010034. Antioxidants (Basel). 2023. PMID: 38247459 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

