Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage
- PMID: 35355864
- PMCID: PMC8958071
- DOI: 10.1155/2022/2453617
Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage
Abstract
Objective: Mitochondrial damage contributes to extracellular matrix (ECM) deposition and renal fibrosis. In this study, we aimed (1) to investigate whether fluorofenidone (AKF-PD) can attenuate mitochondrial damage in two renal fibrosis models: unilateral ureteral obstruction (UUO) and renal ischemia-reperfusion injury (IRI), and (2) to explore the underlying mechanism.
Method: Mitochondrial damage and renal lesions were analyzed in the UUO and IRI models. Mitochondrial energy metabolism, mitochondrial biogenesis, and oxidative stress were measured to assess the effect of AKF-PD on mitochondrial damage and to explore the underlying mechanism. In addition, HK-2 cells were stimulated with TGF-β with and without AKF-PD. The mitochondrial morphology, mtROS, ATP contents, and redox-related proteins were then examined.
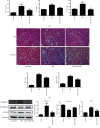
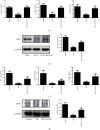
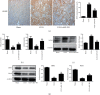
Results: In both UUO and IRI models, AKF-PD relieved renal fibrosis, maintained mitochondrial structure, and increased mitochondrial DNA copy numbers. The protection was associated with (1) sustaining mitochondrial energy metabolism, evident by elevations of tricarboxylic acid (TCA) cycle enzymes and mitochondrial respiratory chain complexes; (2) improving mitochondrial biogenesis with increases of TFAM, NRF1, PGC-1α, and SIRT1; and (3) reducing mitochondrial oxidative stress likely via regulating SOD2, SIRT3, and NOX4 expressions. In HK-2 cells treated with TGF-β, AKF-PD protected mitochondria along with improving mitochondrial morphology, enhancing ATP production, reducing mtROS, and regulating SOD2, SIRT3, and NOX4 expression.
Conclusion: We demonstrate that AKF-PD inhibited renal fibrosis at least in part via protecting mitochondria from damages developed in the UUO and IRI models. The mitochondrial protection was associated with sustaining mitochondrial energy metabolism, improving mitochondrial biogenesis, and reducing mitochondrial oxidative stress. This research verified the protective effect of AKF-PD on mitochondria in the UUO and IRI models and elaborated the underlying mechanism.
Copyright © 2022 Xiaohua Liao et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Fluorofenidone attenuates renal fibrosis by inhibiting the mtROS-NLRP3 pathway in a murine model of folic acid nephropathy.Biochem Biophys Res Commun. 2021 Jan 1;534:694-701. doi: 10.1016/j.bbrc.2020.11.017. Epub 2020 Nov 19. Biochem Biophys Res Commun. 2021. PMID: 33220928
-
Fluorofenidone Attenuates Renal Interstitial Fibrosis by Enhancing Autophagy and Retaining Mitochondrial Function.Cell Biochem Biophys. 2023 Dec;81(4):777-785. doi: 10.1007/s12013-023-01176-7. Epub 2023 Sep 21. Cell Biochem Biophys. 2023. PMID: 37735328
-
Fluorofenidone inhibits apoptosis of renal tubular epithelial cells in rats with renal interstitial fibrosis.Braz J Med Biol Res. 2019 Oct 28;52(11):e8772. doi: 10.1590/1414-431X20198772. eCollection 2019. Braz J Med Biol Res. 2019. PMID: 31664306 Free PMC article.
-
Redox signaling pathways in unilateral ureteral obstruction (UUO)-induced renal fibrosis.Free Radic Biol Med. 2021 Aug 20;172:65-81. doi: 10.1016/j.freeradbiomed.2021.05.034. Epub 2021 May 30. Free Radic Biol Med. 2021. PMID: 34077780 Review.
-
Mitochondrial dysfunction and endoplasmic reticulum stress in the promotion of fibrosis in obstructive nephropathy induced by unilateral ureteral obstruction.Biofactors. 2020 Sep;46(5):716-733. doi: 10.1002/biof.1673. Epub 2020 Sep 9. Biofactors. 2020. PMID: 32905648 Review.
Cited by
-
Dimethyl malonate alleviates obstructive nephropathy by enhancing renal metabolism and inhibiting kidney oxidative stress and inflammation.Front Pharmacol. 2025 Jun 10;16:1530635. doi: 10.3389/fphar.2025.1530635. eCollection 2025. Front Pharmacol. 2025. PMID: 40556756 Free PMC article.
-
Fluorofenidone alleviates liver fibrosis by inhibiting hepatic stellate cell autophagy via the TGF-β1/Smad pathway: implications for liver cancer.PeerJ. 2023 Sep 28;11:e16060. doi: 10.7717/peerj.16060. eCollection 2023. PeerJ. 2023. PMID: 37790613 Free PMC article.
-
Deletion of Pyruvate Carboxylase in Tubular Epithelial Cell Promotes Renal Fibrosis by Regulating SQOR/cGAS/STING-Mediated Glycolysis.Adv Sci (Weinh). 2025 Apr;12(13):e2408753. doi: 10.1002/advs.202408753. Epub 2025 Jan 21. Adv Sci (Weinh). 2025. PMID: 39836535 Free PMC article.
-
Activation of AMPK-PGC-1α pathway ameliorates peritoneal dialysis related peritoneal fibrosis in mice by enhancing mitochondrial biogenesis.Ren Fail. 2022 Dec;44(1):1545-1557. doi: 10.1080/0886022X.2022.2126789. Ren Fail. 2022. PMID: 36148521 Free PMC article.
-
Identification of signature genes for renal ischemia‒reperfusion injury based on machine learning and WGCNA.Heliyon. 2023 Oct 18;9(10):e21151. doi: 10.1016/j.heliyon.2023.e21151. eCollection 2023 Oct. Heliyon. 2023. PMID: 37928383 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

