Strain rate induced toughening of individual collagen fibrils
- PMID: 35355883
- PMCID: PMC8934191
- DOI: 10.1063/5.0084054
Strain rate induced toughening of individual collagen fibrils
Abstract
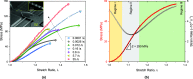
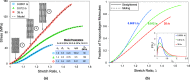
The nonlinear mechanical behavior of individual nanoscale collagen fibrils is governed by molecular stretching and sliding that result in a viscous response, which is still not fully understood. Toward this goal, the in vitro mechanical behavior of individual reconstituted mammalian collagen fibrils was quantified in a broad range of strain-rates, spanning roughly six orders of magnitude, from 10-4 to 35 s-1. It is shown that the nonlinear mechanical response is strain rate sensitive with the tangent modulus in the linear deformation regime increasing monotonically from 214 ± 8 to 358 ± 11 MPa. More pronounced is the effect of the strain rate on the ultimate tensile strength that is found to increase monotonically by a factor of four, from 42 ± 6 to 160 ± 14 MPa. Importantly, fibril strengthening takes place without a reduction in ductility, which results in equivalently large increase in toughness with the increasing strain rate. This experimental strain rate dependent mechanical response is captured well by a structural constitutive model that incorporates the salient features of the collagen microstructure via a process of gradual recruitment of kinked tropocollagen molecules, thus giving rise to the initial "toe-heel" mechanical behavior, followed by molecular stretching and sustained intermolecular slip that is initiated at a strain rate dependent stress threshold. The model shows that the fraction of tropocollagen molecules undergoing straightening increases continuously during loading, whereas molecular sliding is initiated after a small fibril strain (1%-2%) and progressively increases with applied strain.
© 2022 Author(s).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
