Tau and the fractionated default mode network in atypical Alzheimer's disease
- PMID: 35356035
- PMCID: PMC8963312
- DOI: 10.1093/braincomms/fcac055
Tau and the fractionated default mode network in atypical Alzheimer's disease
Abstract
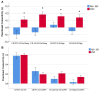
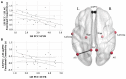
Alzheimer's disease-related atrophy in the posterior cingulate cortex, a key node of the default mode network, is present in the early stages of disease progression across clinical phenotypic variants of the disease. In the typical amnestic variant, posterior cingulate cortex neuropathology has been linked with disrupted connectivity of the posterior default mode network, but it remains unclear if this relationship is observed across atypical variants of Alzheimer's disease. In the present study, we first sought to determine if tau pathology is consistently present in the posterior cingulate cortex and other posterior nodes of the default mode network across the atypical Alzheimer's disease syndromic spectrum. Second, we examined functional connectivity disruptions within the default mode network and sought to determine if tau pathology is related to functional disconnection within this network. We studied a sample of 25 amyloid-positive atypical Alzheimer's disease participants examined with high-resolution MRI, tau (18F-AV-1451) PET, and resting-state functional MRI. In these patients, high levels of tau pathology in the posteromedial cortex and hypoconnectivity between temporal and parietal nodes of the default mode network were observed relative to healthy older controls. Furthermore, higher tau signal and reduced grey matter density in the posterior cingulate cortex and angular gyrus were associated with reduced parietal functional connectivity across individual patients, related to poorer cognitive scores. Our findings converge with what has been reported in amnestic Alzheimer's disease, and together these observations offer a unifying mechanistic feature that relates posterior cingulate cortex tau deposition to aberrant default mode network connectivity across heterogeneous clinical phenotypes of Alzheimer's disease.
Keywords: functional connectivity; logopenic variant primary progressive aphasia; posterior cortical atrophy; resting-state fMRI.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Dickerson BC, Bakkour A, Salat DH, et al. . The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. - PMC - PubMed
-
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
