Effects of polygalacturonase overexpression on pectin distribution in the elongation zones of roots under aluminium stress
- PMID: 35356145
- PMCID: PMC8963292
- DOI: 10.1093/aobpla/plac003
Effects of polygalacturonase overexpression on pectin distribution in the elongation zones of roots under aluminium stress
Abstract
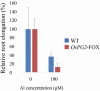
The roots of many plant species contain large amounts of pectin and it contributes to the formation of the rhizosphere. In the present study, the relationship between the root-tip pectin content and aluminium (Al) tolerance in wild-type (WT) and demethylesterified pectin degradation enzyme gene overexpressor (OsPG2-FOX) rice lines was compared. OsPG2-FOX rice showed reduced pectin content in roots, even under control conditions; Al treatment reduced root elongation and the pectin content in the root elongation zone. Wild-type rice showed more pectin accumulation in the root elongation zone after Al treatment. Relative to WT rice, OsPG2-FOX rice showed more Al accumulation in the root elongation zone. These results indicate that the amount of pectin influences Al tolerance and that the distribution of pectin in the root elongation zone inhibits Al accumulation in rice roots. Pectin accumulation in cell walls in the root elongation zone may play a role in protecting rice plants from the Al-induced inhibition of root elongation by regulating pectin distribution.
Keywords: Aluminium; pectin; polygalacturonase; rice (Oryza sativa); root elongation zone.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures





Similar articles
-
Changes in the Distribution of Pectin in Root Border Cells Under Aluminum Stress.Front Plant Sci. 2019 Oct 2;10:1216. doi: 10.3389/fpls.2019.01216. eCollection 2019. Front Plant Sci. 2019. PMID: 31632431 Free PMC article.
-
Structural Alteration of Rice Pectin Affects Cell Wall Mechanical Strength and Pathogenicity of the Rice Blast Fungus Under Weak Light Conditions.Plant Cell Physiol. 2021 Sep 24;62(4):641-649. doi: 10.1093/pcp/pcab019. Plant Cell Physiol. 2021. PMID: 33543762
-
Phosphorus deficiency enhances aluminum tolerance of rice (Oryza sativa) by changing the physicochemical characteristics of root plasma membranes and cell walls.J Plant Physiol. 2014 Jan 15;171(2):9-15. doi: 10.1016/j.jplph.2013.09.012. Epub 2013 Nov 14. J Plant Physiol. 2014. PMID: 24331414
-
Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice.Physiol Plant. 2013 Aug;148(4):502-11. doi: 10.1111/ppl.12005. Epub 2012 Dec 12. Physiol Plant. 2013. PMID: 23136980
-
Boron reduces cell wall aluminum content in rice (Oryza sativa) roots by decreasing H2O2 accumulation.Plant Physiol Biochem. 2019 May;138:80-90. doi: 10.1016/j.plaphy.2019.02.022. Epub 2019 Mar 2. Plant Physiol Biochem. 2019. PMID: 30852240
Cited by
-
OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall.Plants (Basel). 2025 Jun 22;14(13):1912. doi: 10.3390/plants14131912. Plants (Basel). 2025. PMID: 40647921 Free PMC article.
-
Identification and characterization of long noncoding RNAs involved in the aluminum stress response in Medicago truncatula via genome-wide analysis.Front Plant Sci. 2022 Sep 23;13:1017869. doi: 10.3389/fpls.2022.1017869. eCollection 2022. Front Plant Sci. 2022. PMID: 36212300 Free PMC article.
-
Mutation of the Polygalacturonase Gene AcoPG3 Deferred Softening of Pineapple Fruit.Biology (Basel). 2025 Apr 25;14(5):474. doi: 10.3390/biology14050474. Biology (Basel). 2025. PMID: 40427662 Free PMC article.
References
-
- Arroyave C, Tolrà R, Chaves L, de Souza MC, Barceló J, Poschenrieder C. 2018. A proteomic approach to the mechanisms underlying activation of aluminium resistance in roots of Urochloa decumbens. Journal of Inorganic Biochemistry 181:145–151. - PubMed
-
- Baydoun EAH, Abdel-Massih RM, Dani D, Rizk SE, Brett CT. 2001. Galactosyl- and fucosyltransferases in etiolated pea epicotyls: product identification and sub-cellular localisation. Journal of Plant Physiology 158:145–150.
-
- Blumenkrantz N, Gustav AH. 1973. New method for quantitative determination of uronic acids. Analytical Biochemistry 54:484–489. - PubMed
-
- Chang YC, Yamamoto Y, Matsumoto H. 1999. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell and Environment 22:1009–1017.
LinkOut - more resources
Full Text Sources

