Enhanced sensitivity of an Ah-receptor system in yeast through condition modification and use of mammalian modifiers
- PMID: 35356645
- PMCID: PMC8958262
- DOI: 10.1016/j.toxrep.2022.03.012
Enhanced sensitivity of an Ah-receptor system in yeast through condition modification and use of mammalian modifiers
Abstract
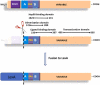
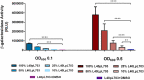
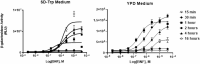
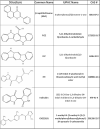
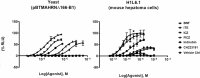
Proteins, such as the Ah receptor (AHR), hold potential as sensors to detect ligands in environmental and biological samples, and may also serve as tools to regulate biosynthetic and industrial processes. The AHR is also a prototype system for the PAS superfamily that can sense and mediate adaptation to signals as diverse as light, voltage, oxygen and an array of small molecules. The yeast, S. cerevisiae, has proven to be an important model to study the signal transduction of sensors like the AHR because of its ease of use, numerous available strategies for genetic manipulation, and capacity for heterologous expression. To better understand the utility of sensor proteins as components of yeast detection systems, we characterized a chimeric AHR-LexA system that drives expression from a Lex operator (LexO) driven, beta-galactosidase (β-Gal) reporter. In this report, we demonstrate that improvements in assays sensitivity and pharmacology can arise from the careful optimization of yeast growth phase and the duration of ligand exposure. We also report that the coexpression of heterotypic modifiers from mammalian cells (e.g., the ARA9 and ARA3 proteins), can improve yeast assay performance. We propose that complementing these assay improvements with previously reported yeast mutations described by others will expand the utility of the AHR for biotechnology applications.
Keywords: Ah receptor; Assay; Ligands; Polycyclic aromatic hydrocarbons; Reporter; Yeast.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
The 90-kDa heat shock protein is essential for Ah receptor signaling in a yeast expression system.J Biol Chem. 1994 Dec 2;269(48):30109-12. J Biol Chem. 1994. PMID: 7982913
-
ARA9 modifies agonist signaling through an increase in cytosolic aryl hydrocarbon receptor.J Biol Chem. 2000 Mar 3;275(9):6153-9. doi: 10.1074/jbc.275.9.6153. J Biol Chem. 2000. PMID: 10692406
-
Development of a recombinant yeast assay to detect ah-receptor ligands.Toxicol Mech Methods. 2006;16(5):287-94. doi: 10.1080/15376520600616875. Toxicol Mech Methods. 2006. PMID: 20021027
-
A dynamic role for the Ah receptor in cell signaling? Insights from a diverse group of Ah receptor interacting proteins.J Biochem Mol Toxicol. 2002;16(6):317-25. doi: 10.1002/jbt.10051. J Biochem Mol Toxicol. 2002. PMID: 12481307 Review.
-
Rodent genetic models of Ah receptor signaling.Drug Metab Rev. 2021 Aug;53(3):350-374. doi: 10.1080/03602532.2021.1955916. Epub 2021 Aug 25. Drug Metab Rev. 2021. PMID: 34289754 Free PMC article. Review.
References
-
- Barr M.M. Super models. Physiol. Genomics. 2003;13(1):15–24. - PubMed
-
- Cox M.B., Miller C.A. The p23 co-chaperone facilitates dioxin receptor signaling in a yeast model system. Toxicol. Lett. 2002;129(1):13–21. - PubMed
-
- Ma Q., Whitlock J.P., Jr. A novel cytoplasmic protein that interacts with the ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-Tetrachlorodibenzo-<em>p</em>-dioxin *. J. Biolo. Chem. 1997;272(14):8878–8884. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

