Genetic or siRNA inhibition of MBD2 attenuates the UUO- and I/R-induced renal fibrosis via downregulation of EGR1
- PMID: 35356685
- PMCID: PMC8933641
- DOI: 10.1016/j.omtn.2022.02.015
Genetic or siRNA inhibition of MBD2 attenuates the UUO- and I/R-induced renal fibrosis via downregulation of EGR1
Abstract
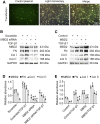
DNA methylation plays a pivotal role in the progression of renal fibrosis. Methyl-CpG-binding domain protein 2 (MBD2), a protein reader of methylation, is involved in the development of acute kidney injury (AKI) caused by vancomycin. However, the role and mechanism of action of MBD2 in renal remain unclear. In this study, MBD2 mediated extracellular matrix (ECM) production induced by TGF-β1 in Boston University mouse proximal tubule (BUMPT) cells,and upregulated the expression EGR1 to promote ECM production in murine embryonic NIH 3T3 fibroblasts. ChIP analysis demonstrated that MBD2 physically interacted with the promoter region of the CpG islands of EGR1 genes and then activated their expression by inducing hypomethylation of the promoter region. In vivo, PT-MBD2-KO attenuated unilateral ureteral obstruction (UUO)-induced renal tubulointerstitial fibrosis via downregulation of EGR1, which was demonstrated by the downregulation of fibronectin (FN), collagen I and IV, α-SMA, and EGR1. Injection of MBD2-siRNA attenuated the UUO- and I/R-induced renal fibrosis. Those molecular changes were verified by biopsies from patients with obstructive nephropathy (OB). These data collectively demonstrated that inhibition of MBD2 reduces renal fibrosis via downregulating EGR1, which could be a target for treatment of fibrotic kidney disease.
Keywords: EGR1; I/R; MBD2; MT: Bioinformatics; UUO; renal fibrosis.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Methyl-CpG-binding domain protein 2 contributes to renal fibrosis through promoting polarized M1 macrophages.Cell Death Dis. 2022 Feb 8;13(2):125. doi: 10.1038/s41419-022-04577-3. Cell Death Dis. 2022. PMID: 35136032 Free PMC article.
-
MBD2 Mediates Septic AKI through Activation of PKCη/p38MAPK and the ERK1/2 Axis.Mol Ther Nucleic Acids. 2020 Sep 28;23:76-88. doi: 10.1016/j.omtn.2020.09.028. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2020. PMID: 33335794 Free PMC article.
-
Intermedin is upregulated and attenuates renal fibrosis by inhibition of oxidative stress in rats with unilateral ureteral obstruction.Nephrology (Carlton). 2015 Nov;20(11):820-31. doi: 10.1111/nep.12520. Nephrology (Carlton). 2015. PMID: 26014968
-
MBD2 mediates renal cell apoptosis via activation of Tox4 during rhabdomyolysis-induced acute kidney injury.J Cell Mol Med. 2021 May;25(10):4562-4571. doi: 10.1111/jcmm.16207. Epub 2021 Mar 25. J Cell Mol Med. 2021. PMID: 33764669 Free PMC article.
-
Astragaloside IV inhibits renal tubulointerstitial fibrosis by blocking TGF-β/Smad signaling pathway in vivo and in vitro.Exp Biol Med (Maywood). 2014 Oct;239(10):1310-24. doi: 10.1177/1535370214532597. Epub 2014 May 30. Exp Biol Med (Maywood). 2014. PMID: 24879422
Cited by
-
Computational recognition of regulator genes and signature for ferroptosis with implications on immunological properties and clinical management of atopic dermatitis.Front Immunol. 2024 Sep 6;15:1412382. doi: 10.3389/fimmu.2024.1412382. eCollection 2024. Front Immunol. 2024. PMID: 39308857 Free PMC article.
-
Gpx3 and Egr1 Are Involved in Regulating the Differentiation Fate of Cardiac Fibroblasts under Pressure Overload.Oxid Med Cell Longev. 2022 Jun 28;2022:3235250. doi: 10.1155/2022/3235250. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35799890 Free PMC article.
-
LncRNA 148400 Promotes the Apoptosis of Renal Tubular Epithelial Cells in Ischemic AKI by Targeting the miR-10b-3p/GRK4 Axis.Cells. 2022 Dec 9;11(24):3986. doi: 10.3390/cells11243986. Cells. 2022. PMID: 36552750 Free PMC article.
-
mmu-lncRNA 121686/hsa-lncRNA 520657 induced by METTL3 drive the progression of AKI by targeting miR-328-5p/HtrA3 signaling axis.Mol Ther. 2022 Dec 7;30(12):3694-3713. doi: 10.1016/j.ymthe.2022.07.014. Epub 2022 Jul 21. Mol Ther. 2022. PMID: 35869629 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors alleviate renal fibrosis in diabetic kidney disease by inhibiting Hmgcs2 and Btg2 in proximal tubular cells.J Transl Med. 2025 Aug 25;23(1):959. doi: 10.1186/s12967-025-06788-6. J Transl Med. 2025. PMID: 40855324 Free PMC article.
References
-
- Hamidi T., Singh A.K., Chen T. Genetic alterations of DNA methylation machinery in human diseases. Epigenomics. 2015;7:247–265. - PubMed
-
- Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. - PubMed
-
- Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. - PubMed
-
- Larkin B.P., Glastras S.J., Chen H., Pollock C.A., Saad S. DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J. 2018;32:5215–5226. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

