A Biofabrication Strategy for a Custom-Shaped, Non-Synthetic Bone Graft Precursor with a Prevascularized Tissue Shell
- PMID: 35356783
- PMCID: PMC8959609
- DOI: 10.3389/fbioe.2022.838415
A Biofabrication Strategy for a Custom-Shaped, Non-Synthetic Bone Graft Precursor with a Prevascularized Tissue Shell
Abstract
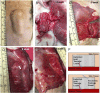
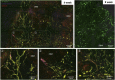
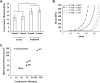
Critical-sized defects of irregular bones requiring bone grafting, such as in craniofacial reconstruction, are particularly challenging to repair. With bone-grafting procedures growing in number annually, there is a reciprocal growing interest in bone graft substitutes to meet the demand. Autogenous osteo(myo)cutaneous grafts harvested from a secondary surgical site are the gold standard for reconstruction but are associated with donor-site morbidity and are in limited supply. We developed a bone graft strategy for irregular bone-involved reconstruction that is customizable to defect geometry and patient anatomy, is free of synthetic materials, is cellularized, and has an outer pre-vascularized tissue layer to enhance engraftment and promote osteogenesis. The graft, comprised of bioprinted human-derived demineralized bone matrix blended with native matrix proteins containing human mesenchymal stromal cells and encased in a simple tissue shell containing isolated, human adipose microvessels, ossifies when implanted in rats. Ossification follows robust vascularization within and around the graft, including the formation of a vascular leash, and develops mechanical strength. These results demonstrate an early feasibility animal study of a biofabrication strategy to manufacture a 3D printed patient-matched, osteoconductive, tissue-banked, bone graft without synthetic materials for use in craniofacial reconstruction. The bone fabrication workflow is designed to be performed within the hospital near the Point of Care.
Keywords: 3D bioprinting; bone; craniofacial; ossification; patient-specific; reconstruction; tissue fabrication; vascularization.
Copyright © 2022 Moss, Ortiz-Hernandez, Levin, Richburg, Gerton, Cook, Houlton, Rizvi, Goodwin, Golway, Ripley and Hoying.
Conflict of interest statement
Authors SM, TG, MC, MG, and JH were employed by the company Advanced Solutions Life Sciences. Author PG was employed by the company Cytiva. JH and MG are equity holders in Advanced Solutions Life Sciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

