Naloxegol to Prevent Constipation in ICU Adults Receiving Opioids: A Randomized Double-Blind Placebo-Controlled Pilot Trial
- PMID: 35356796
- PMCID: PMC8958087
- DOI: 10.1155/2022/7541378
Naloxegol to Prevent Constipation in ICU Adults Receiving Opioids: A Randomized Double-Blind Placebo-Controlled Pilot Trial
Abstract
Background: Constipation is frequent in critically ill adults receiving opioids. Naloxegol (N), a peripherally acting mu-receptor antagonist (PAMORA), may reduce constipation. The objective of this trial was to evaluate the efficacy and safety of N to prevent constipation in ICU adults receiving opioids. Methods and Patients. In this single-center, double-blind, randomized trial, adults admitted to a medical ICU receiving IV opioids (≥100 mcg fentanyl/day), and not having any of 17 exclusion criteria, were randomized to N (25 mg) or placebo (P) daily randomized to receive N (25mg) or placebo (P) and docusate 100 mg twice daily until ICU discharge, 10 days, or diarrhea (≥3 spontaneous bowel movement (SBM)/24 hours) or a serious adverse event related to study medication. A 4-step laxative protocol was initiated when there was no SBM ≥3 days.
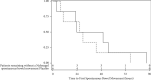
Results: Only 318 (20.6%) of the 1542 screened adults during the 1/17-10/19 enrolment period met all inclusion criteria. Of these, only 19/381 (4.9%) met all eligibility criteria. After 7 consent refusals, 12 patients were randomized. The study was stopped early due to enrolment futility. The N (n = 6) and P (n = 6) groups were similar. The time to first SBM (N 41.4 ± 31.7 vs. P 32.5 ± 25.4 hours, P = 0.56) was similar. The maximal daily abdominal pressure was significantly lower in the N group (N 10 ± 4 vs. P 13 ± 5, P = 0.002). The median (IQR) daily SOFA scores were higher in N (N 7 (4, 8) vs. P 4 (3, 5), P < 0.001). Laxative protocol use was similar (N 83.3% vs. P 66.6%; P = 0.51). Diarrhea prevalence was high but similar (N 66.6% vs. P 66.6%; P = 1.0). No patient experienced opioid withdrawal.
Conclusions: Important recruitment challenges exist for ICU trials evaluating the use of PAMORAs for constipation prevention. Despite being underpowered, our results suggest time to first SBM with naloxegol, if different than P, may be small. The effect of naloxegol on abdominal pressure, SOFA, and the interaction between the two requires further research.
Copyright © 2022 Matthew S. Duprey et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures

References
-
- Devlin J. W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Critical Care Medicine . 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. - DOI - PubMed
-
- Reintam Blaser A., Malbrain M. L. N. G., Starkopf J., et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Medicine . 2012;38(3):384–394. doi: 10.1007/s00134-011-2459-y. - DOI - PMC - PubMed
-
- Mostafa S. M., Bhandari S., Ritchie G., Gratton N., Wenstone R. Constipation and its implications in the critically ill patient † †an abstract of a part of this paper has been presented at the European society of intensive care medicine in Rome, october 2000 (published in intensive care medicine: mostafa sm and ritchie G. Failure to wean critically ill patients from mechanical ventilation due to constipation (A). Intensive care medicine 2000, 26 (suppl. 3): S336), and at the intensive care society meeting in london, december 2000 (published in the British journal of anaesthesia: mostafa sm, bhandari S, ritchie G. Constipation and its implications in the critically ill: a national survey of United Kingdom intensive care units (A). British journal of anaesthesia 2001; 87: 343P) British Journal of Anaesthesia . 2003;91(6):815–819. doi: 10.1093/bja/aeg275. - DOI - PubMed
-
- Floettmann E., Bui K., Sostek M., Payza K., Eldon M. Pharmacologic profile of naloxegol, a peripherally acting µ-opioid receptor antagonist, for the treatment of opioid-induced constipation. Journal of Pharmacology and Experimental Therapeutics . 2017;361(2):280–291. doi: 10.1124/jpet.116.239061. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

