Age-specific reference values for the 5th generation cardiac troponin T assay in Chinese children
- PMID: 35356945
- PMCID: PMC10684185
- DOI: 10.1097/MD.0000000000029101
Age-specific reference values for the 5th generation cardiac troponin T assay in Chinese children
Abstract
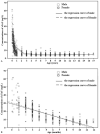
The clinical use of the cardiac troponin T (cTnT) assay was limited to the adult population in the diagnosis and prognosis of myocardial injury. However, emerging studies indicated its significant value in the assessment of pediatric cardiology, and it has been routinely measured in most hospitals. Our study investigated the normative values of cTnT in Chinese children and reported the age-specific 99th percentile cut-off for them.A total of 1280 apparently healthy Chinese children were enrolled in our study. Serum levels of cTnT were analyzed on the Roche Elecsys Troponin T Gen 5 STAT assay. According to the Clinical and Laboratory Standards Institute C28-A3 guideline, the 99th percentile upper reference limits (URLs) with 90% confidence intervals (CIs) were calculated in different age subgroups.The 99th percentile URL was 38 (90%CI: 37.0-51.0) ng/L for 1 to <4months old, 26 (90%CI: 25.2-28.5) ng/L for 4 to ≤ 12months old, and 12 (90%CI: 11.1-12.9) ng/L for 1 to 18 years old, respectively. For subjects aged from 1 to 18years, boys had slightly higher cTnT levels than girls (P = .003), while our assay could not measure low cTnT concentrations (≥the limit of detection) in 50% girls.Our study provided age-specific URLs of cTnT for Chinese children, with the 5th generation cTnT assay from Roche Diagnostics. It had significant clinical implications in the interpretation and use of test results for pediatric cardiology.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation 2018;138:e618- 51. - PubMed
-
- Wu AHB, Christenson RH, Greene DN, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645- 55. - PubMed
-
- Clerico A, Zaninotto M, Padoan A, et al. Evaluation of analytical performance of immunoassay methods for cTnI and cTnT: from theory to practice. Adv Clin Chem 2019;93:239- 62. - PubMed
-
- Collinson PO, Saenger AK, Apple FS. IFCC C-CBHigh sensitivity, contemporary and point-of-care cardiac troponin assays: educational aids developed by the IFCC Committee on Clinical Application of Cardiac BioMarkers. Clin Chem Lab Med 2019;57:623- 32. - PubMed
-
- Mueller C, Giannitsis E, Christ M, et al. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med 2016;68:76- 87.e4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

