Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months
- PMID: 35357044
- PMCID: PMC10325874
- DOI: 10.1111/xen.12744
Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months
Abstract
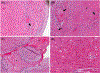
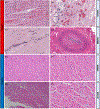
We report orthotopic (life-supporting) survival of genetically engineered porcine cardiac xenografts (with six gene modifications) for almost 9 months in baboon recipients. This work builds on our previously reported heterotopic cardiac xenograft (three gene modifications) survival up to 945 days with an anti-CD40 monoclonal antibody-based immunosuppression. In this current study, life-supporting xenografts containing multiple human complement regulatory, thromboregulatory, and anti-inflammatory proteins, in addition to growth hormone receptor knockout (KO) and carbohydrate antigen KOs, were transplanted in the baboons. Selective "multi-gene" xenografts demonstrate survival greater than 8 months without the requirement of adjunctive medications and without evidence of abnormal xenograft thickness or rejection. These data demonstrate that selective "multi-gene" modifications improve cardiac xenograft survival significantly and may be foundational for paving the way to bridge transplantation in humans.
Keywords: CRISPR; cardiac xenotransplantation; heart failure; pig heart; xenotransplantation.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
David Ayares, Will Eyestone, Amy Dandro, Kasinath Kuravi, Lori Sorrels, and Todd Vaught are employed by Revivicor/United Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







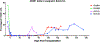
References
-
- Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. - PubMed
-
- Yamamoto T, Hara H, Foote J, et al. Life-supporting kidney xenotransplantation from genetically engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation. 2019;103:2090–2104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

