The Mechanism of Pertussis Cough Revealed by the Mouse-Coughing Model
- PMID: 35357202
- PMCID: PMC9040802
- DOI: 10.1128/mbio.03197-21
The Mechanism of Pertussis Cough Revealed by the Mouse-Coughing Model
Erratum in
-
Correction for Hiramatsu et al., "The Mechanism of Pertussis Cough Revealed by the Mouse-Coughing Model".mBio. 2022 Jun 28;13(3):e0144622. doi: 10.1128/mbio.01446-22. Epub 2022 May 31. mBio. 2022. PMID: 35638745 Free PMC article. No abstract available.
Retraction in
-
Retraction for Hiramatsu et al., "The Mechanism of Pertussis Cough Revealed by the Mouse-Coughing Model".mBio. 2025 Mar 12;16(3):e0392124. doi: 10.1128/mbio.03921-24. Epub 2025 Feb 5. mBio. 2025. PMID: 39907486 Free PMC article. No abstract available.
Abstract
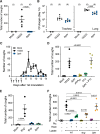
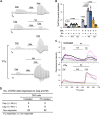
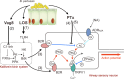
Pertussis, also known as whooping cough, is a contagious respiratory disease caused by the Gram-negative bacterium Bordetella pertussis. This disease is characterized by severe and uncontrollable coughing, which imposes a significant burden on patients. However, its etiological agent and the mechanism are totally unknown because of a lack of versatile animal models that reproduce the cough. Here, we present a mouse model that reproduces coughing after intranasal inoculation with the bacterium or its components and demonstrate that lipooligosaccharide (LOS), pertussis toxin (PTx), and Vag8 of the bacterium cooperatively function to cause coughing. Bradykinin induced by LOS sensitized a transient receptor potential ion channel, TRPV1, which acts as a sensor to evoke the cough reflex. Vag8 further increased bradykinin levels by inhibiting the C1 esterase inhibitor, the major downregulator of the contact system, which generates bradykinin. PTx inhibits intrinsic negative regulation systems for TRPV1 through the inactivation of Gi GTPases. Our findings provide a basis to answer long-standing questions on the pathophysiology of pertussis cough. IMPORTANCE The Gram-negative bacterium Bordetella pertussis causes a respiratory disease called whooping cough, or pertussis. This disease is characterized by paroxysmal coughing, the mechanism of which has not been intensively studied because of a lack of versatile animal models that reproduce the cough. In this study, we present a mouse model that reproduces coughing after intranasal inoculation with the bacterium or its components. Using this model, we demonstrate that lipooligosaccharide, Vag8, and pertussis toxin of the bacteria cooperatively function to cause coughing. Our results also indicate that bradykinin, an inflammatory mediator, and TRPV1, an ion channel linked to nociceptive signaling, are host factors involved in the coughing mechanism.
Keywords: Bordetella pertussis; TRPV1; Vag8; bradykinin; cough; pertussis toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
What Causes the Cough in Whooping Cough?mBio. 2022 Jun 28;13(3):e0091722. doi: 10.1128/mbio.00917-22. Epub 2022 May 23. mBio. 2022. PMID: 35604095 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

