Effect of Patient-Directed Messaging on Colorectal Cancer Screening: A Randomized Clinical Trial
- PMID: 35357457
- PMCID: PMC8972032
- DOI: 10.1001/jamanetworkopen.2022.4529
Effect of Patient-Directed Messaging on Colorectal Cancer Screening: A Randomized Clinical Trial
Abstract
Importance: Colorectal cancer (CRC) screening is underused in the US. Tailored message interventions have shown benefit for increasing screening uptake of mammography and Papanicolaou testing, but their role in CRC screening is less clear.
Objective: To evaluate the effectiveness of a tailored message telephone intervention prior to scheduling of a screening or surveillance colonoscopy and its effect on CRC screening completion rates.
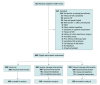
Design, setting, and participants: This randomized clinical trial was conducted from July 2017 through August 2018 at the University of Pennsylvania Health System (UPHS), an urban academic medical center. Participants were asymptomatic patients aged 50 to 75 years who were eligible for CRC screening or surveillance, had been referred for colonoscopy, and did not have a scheduled colonoscopy appointment. Data analysis was conducted from January to September 2019.
Interventions: Patients underwent block randomization in a 1:1:1 ratio to 1 of 3 study arms. Participants in the usual care group were contacted via a mailed letter and instructed to call to schedule a colonoscopy. In the generic message group, participants were contacted by telephone, completed an assessment, and received a uniform, nontailored message encouraging colonoscopy scheduling. Participants in the tailored message group were contacted by telephone, completed an assessment, and received a tailored message encouraging colonoscopy scheduling based on their identified assessment cohort.
Main outcomes and measures: The primary outcome was colonoscopy completion rate within 120 days of enrollment. The secondary outcome was colonoscopy scheduling rate appointment within 120 days of enrollment.
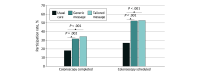
Results: A total of 600 participants (median [IQR] age, 56 [51-63] years; 373 women [62.2%]) were enrolled, including 200 participants randomized to usual care, 200 participants randomized to the generic message, and 200 participants randomized to the tailored message. The total sample included 12 Asian participants (2.0%), 324 Black participants (54.0%), and 227 White participants (37.8%), and 9 participants (1.5%) were of Latino or Hispanic ethnicity. Colonoscopy completion was significantly higher for both the tailored message group (69 participants [34.5%]) and the generic message group (64 participants [32.0%]) compared with the usual care group (37 participants [18.5%]) (P < .001 and P = .002, respectively). Scheduling rates were also significantly higher in both groups, with 106 participants (53.0%) in the tailored message group and 105 participants (52.5%) in the generic message group scheduling appointments, compared with 54 participants (27.0%) in the usual care arm (P < .001 for both).
Conclusions and relevance: In this randomized clinical trial among individuals whose CRC screening was not up to date, both a tailored message intervention and a generic message intervention were significantly more effective at increasing colonoscopy scheduling and completion rates compared with usual care. These findings suggest that individualized health communications can increase individual motivation to obtain CRC screening.
Trial registration: ClinicalTrials.gov Identifier: NCT03310892.
Conflict of interest statement
Figures
Similar articles
-
Patient Navigation Plus Tailored Digital Video Disc Increases Colorectal Cancer Screening Among Low-Income and Minority Patients Who Did Not Attend a Scheduled Screening Colonoscopy: A Randomized Trial.Ann Behav Med. 2024 Apr 11;58(5):314-327. doi: 10.1093/abm/kaae013. Ann Behav Med. 2024. PMID: 38470961 Free PMC article. Clinical Trial.
-
Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial.JAMA Intern Med. 2013 Oct 14;173(18):1725-32. doi: 10.1001/jamainternmed.2013.9294. JAMA Intern Med. 2013. PMID: 23921906 Free PMC article. Clinical Trial.
-
Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial.JAMA. 2017 Sep 5;318(9):806-815. doi: 10.1001/jama.2017.11389. JAMA. 2017. PMID: 28873161 Free PMC article. Clinical Trial.
-
Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-analysis.JAMA Intern Med. 2018 Dec 1;178(12):1645-1658. doi: 10.1001/jamainternmed.2018.4637. JAMA Intern Med. 2018. PMID: 30326005 Free PMC article.
-
Addition of Financial Incentives to Mailed Outreach for Promoting Colorectal Cancer Screening: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Aug 2;4(8):e2122581. doi: 10.1001/jamanetworkopen.2021.22581. JAMA Netw Open. 2021. PMID: 34432010 Free PMC article.
Cited by
-
Personalizing Communication of Clinicians with Chronically Ill Elders in Digital Encounters-A Patient-Centered View.Healthcare (Basel). 2024 Feb 8;12(4):434. doi: 10.3390/healthcare12040434. Healthcare (Basel). 2024. PMID: 38391809 Free PMC article.
-
Socioeconomic differences in discrepancies between expected and experienced discomfort from colonoscopy and colon capsule endoscopy.Heliyon. 2024 Jul 8;10(14):e34274. doi: 10.1016/j.heliyon.2024.e34274. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39100485 Free PMC article.
-
Interventions to Improve Endoscopic Screening Adherence of Cancer in High-Risk Populations: A Scoping Review.Patient Prefer Adherence. 2024 Mar 20;18:709-720. doi: 10.2147/PPA.S443607. eCollection 2024. Patient Prefer Adherence. 2024. PMID: 38524198 Free PMC article.
-
Nationwide, Pragmatic, Direct-to-Patient Primary Aldosteronism Testing Program.Hypertension. 2025 Jun;82(6):977-988. doi: 10.1161/HYPERTENSIONAHA.125.24648. Epub 2025 Feb 21. Hypertension. 2025. PMID: 39981578
-
Ten-year multicentric retrospective analysis regarding postoperative complications and impact of comorbidities in hemorrhoidal surgery with literature review.World J Clin Cases. 2023 Jan 16;11(2):366-384. doi: 10.12998/wjcc.v11.i2.366. World J Clin Cases. 2023. PMID: 36686344 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous