Hyperandrogenism diminishes maternal-fetal fatty acid transport by increasing FABP4-mediated placental lipid accumulation†
- PMID: 35357467
- PMCID: PMC9614932
- DOI: 10.1093/biolre/ioac059
Hyperandrogenism diminishes maternal-fetal fatty acid transport by increasing FABP4-mediated placental lipid accumulation†
Abstract
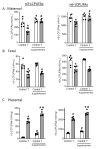
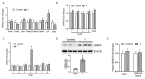
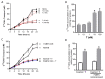
Long-chain polyunsaturated fatty acids (LCPUFAs) are critical for fetal brain development. Infants born to preeclamptic mothers or those born growth restricted due to placental insufficiency have reduced LCPUFA and are at higher risk for developing neurodevelopmental disorders. Since plasma levels of testosterone (T) and fatty acid-binding protein 4 (FABP4) are elevated in preeclampsia, we hypothesized that elevated T induces the expression of FABP4 in the placenta leading to compromised transplacental transport of LCPUFAs. Increased maternal T in pregnant rats significantly decreased n-3 and n-6 LCPUFA levels in maternal and fetal circulation, but increased their placental accumulation. Dietary LCPUFAs supplementation in T dams increased LCPUFA levels in the maternal circulation and further augmented placental storage, while failing to increase fetal levels. The placenta in T dams exhibited increased FABP4 mRNA and protein levels. In vitro, T dose-dependently upregulated FABP4 transcription in trophoblasts. Testosterone stimulated androgen receptor (AR) recruitment to the androgen response element and trans-activated FABP4 promoter activity, both of which were abolished by AR antagonist. Testosterone in pregnant rats and cultured trophoblasts significantly reduced transplacental transport of C14-docosahexaenoic acid (DHA) and increased C14-DHA accumulation in the placenta. Importantly, FABP4 overexpression by itself in pregnant rats and trophoblasts increased transplacental transport of C14-DHA with no significant placental accumulation. Testosterone exposure, in contrast, inhibited this FABP4-mediated effect by promoting C14-DHA placental accumulation.
Keywords: FABP4; androgens; fatty acids; fetal growth; placenta; preeclampsia; pregnancy.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Kremmyda LS, Tvrzicka E, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease: a review. Part 2: fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2011; 155:195–218. - PubMed
-
- Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr 2003; 143:S1–S8. - PubMed
-
- Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr 2010; 30:237–255. - PubMed
-
- Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta 2002; 23:S28–S38. - PubMed
-
- Dunlop M, Court JM. Lipogenesis in developing human adipose tissue. Early Hum Dev 1978; 2:123–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

