APYRASE1/2 mediate red light-induced de-etiolation growth in Arabidopsis seedlings
- PMID: 35357495
- PMCID: PMC9237676
- DOI: 10.1093/plphys/kiac150
APYRASE1/2 mediate red light-induced de-etiolation growth in Arabidopsis seedlings
Abstract
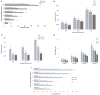
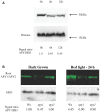
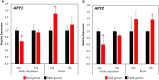
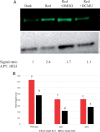
In etiolated seedlings, red light (R) activates phytochrome and initiates signals that generate major changes at molecular and physiological levels. These changes include inhibition of hypocotyl growth and promotion of the growth of primary roots, apical hooks, and cotyledons. An earlier report showed that the sharp decrease in hypocotyl growth rapidly induced by R was accompanied by an equally rapid decrease in the transcript and protein levels of two closely related apyrases (APYs; nucleoside triphosphate-diphosphohydrolases) in Arabidopsis (Arabidopsis thaliana), APY1 and APY2, enzymes whose expression alters auxin transport and growth in seedlings. Here, we report that single knockouts of either APY inhibit R-induced promotion of the growth of primary roots, apical hooks, and cotyledons, and RNAi-induced suppression of APY1 expression in the background of apy2 inhibits R-induced apical hook opening. When R-irradiated primary roots and apical hook-cotyledons began to show a gradual increase in their growth relative to dark controls, they concurrently showed increased levels of APY protein, but in hook-cotyledon tissue, this occurred without parallel increases in their transcripts. In wild-type seedlings whose root growth is suppressed by the photosynthesis inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea, the R-induced increased APY expression in roots was also inhibited. In unirradiated plants, the constitutive expression of APY2 promoted both hook opening and changes in the transcript abundance of Small Auxin Upregulated RNA (SAUR), SAUR17 and SAUR50 that help mediate de-etiolation. These results provide evidence that the expression of APY1/APY2 is regulated by R and that APY1/APY2 participate in the signaling pathway by which phytochrome induces differential growth changes in different tissues of etiolated seedlings.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures

Similar articles
-
SAUR17 and SAUR50 Differentially Regulate PP2C-D1 during Apical Hook Development and Cotyledon Opening in Arabidopsis.Plant Cell. 2020 Dec;32(12):3792-3811. doi: 10.1105/tpc.20.00283. Epub 2020 Oct 22. Plant Cell. 2020. PMID: 33093148 Free PMC article.
-
Role for apyrases in polar auxin transport in Arabidopsis.Plant Physiol. 2012 Dec;160(4):1985-95. doi: 10.1104/pp.112.202887. Epub 2012 Oct 15. Plant Physiol. 2012. PMID: 23071251 Free PMC article.
-
The Transcription Factors TCP4 and PIF3 Antagonistically Regulate Organ-Specific Light Induction of SAUR Genes to Modulate Cotyledon Opening during De-Etiolation in Arabidopsis.Plant Cell. 2019 May;31(5):1155-1170. doi: 10.1105/tpc.18.00803. Epub 2019 Mar 25. Plant Cell. 2019. PMID: 30914467 Free PMC article.
-
Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk.Planta. 2017 Mar;245(3):467-489. doi: 10.1007/s00425-017-2651-6. Epub 2017 Feb 10. Planta. 2017. PMID: 28188422 Review.
-
Environmental Control of Hypocotyl Elongation.Annu Rev Plant Biol. 2024 Jul;75(1):489-519. doi: 10.1146/annurev-arplant-062923-023852. Epub 2024 Jul 2. Annu Rev Plant Biol. 2024. PMID: 38012051 Review.
Cited by
-
Overexpression of PagSTOMAGEN, a Positive Regulator of Stomatal Density, Promotes Vegetative Growth in Poplar.Int J Mol Sci. 2022 Sep 5;23(17):10165. doi: 10.3390/ijms231710165. Int J Mol Sci. 2022. PMID: 36077563 Free PMC article.
-
A matter of time: Red light regulation of APYRASES shapes seedling growth during de-etiolation.Plant Physiol. 2022 Jun 27;189(3):1202-1203. doi: 10.1093/plphys/kiac188. Plant Physiol. 2022. PMID: 35460245 Free PMC article. No abstract available.
-
Role of calcium in regulating key steps in phytochrome-induced signaling pathways.Physiol Mol Biol Plants. 2023 Dec;29(12):1875-1879. doi: 10.1007/s12298-023-01403-8. Epub 2023 Dec 27. Physiol Mol Biol Plants. 2023. PMID: 38222279 Free PMC article. Review.
-
ATP homeostasis and signaling in plants.Plant Commun. 2024 Apr 8;5(4):100834. doi: 10.1016/j.xplc.2024.100834. Epub 2024 Feb 7. Plant Commun. 2024. PMID: 38327057 Free PMC article. Review.
-
Genome-wide identification and analyses of ZmAPY genes reveal their roles involved in maize development and abiotic stress responses.Mol Breed. 2024 May 13;44(5):37. doi: 10.1007/s11032-024-01474-9. eCollection 2024 May. Mol Breed. 2024. PMID: 38745883 Free PMC article.
References
-
- Casal JJ, Mella A, Ballare CL, Maldonado S (2006) Phytochrome-mediated effects on extracellular peroxidase activity, lignin content and bending resistance in etiolated Vicia faba epicotyls. Physiol Plant 92:555–562
-
- Clark GB, Morgan RO, Fernandez MP, Salmi ML, Roux SJ (2014) Breakthroughs spotlighting roles for extracellular nucleotides and apyrases in stress responses and growth and development. Plant Sci 225:107–116 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

