Hyperthermic Intraperitoneal Chemotherapy-Induced Molecular Changes in Humans Validate Preclinical Data in Ovarian Cancer
- PMID: 35357903
- PMCID: PMC8984280
- DOI: 10.1200/PO.21.00239
Hyperthermic Intraperitoneal Chemotherapy-Induced Molecular Changes in Humans Validate Preclinical Data in Ovarian Cancer
Erratum in
-
Erratum.JCO Precis Oncol. 2022 Jun;6:e2200296. doi: 10.1200/PO.22.00296. JCO Precis Oncol. 2022. PMID: 35709405 Free PMC article. No abstract available.
Abstract
Purpose: Hyperthermic intraperitoneal chemotherapy (HIPEC) confers a survival benefit in epithelial ovarian cancer (EOC) and in preclinical models. However, the molecular changes induced by HIPEC have not been corroborated in humans.
Patients and methods: A feasibility trial evaluated clinical and safety outcomes of HIPEC with cisplatin during optimal cytoreductive surgery (CRS) in patients with EOC diagnosed with stage III, IV, or recurrent EOC. Pre- and post-HIPEC biopsies were comprehensively profiled with genomic and transcriptomic sequencing to identify mutational and RNAseq signatures correlating with response; the tumor microenvironment was profiled to identify potential immune biomarkers; and transcriptional signatures of tumors and normal samples before and after HIPEC were compared to investigate HIPEC-induced acute transcriptional changes.
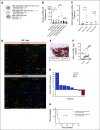
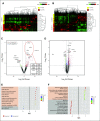
Results: Thirty-five patients had HIPEC at the time of optimal CRS; all patients had optimal CRS. The median progression-free survival (PFS) was 24.7 months for primary patients and 22.4 for recurrent patients. There were no grade 4 or 5 adverse events. Anemia was the most common grade 3 adverse event (43%). Hierarchical cluster analyses identified distinct transcriptomic signatures of good versus poor responders to HIPEC correlating with a PFS of 29.9 versus 7.3 months, respectively. Among good responders, significant HIPEC-induced molecular changes included immune pathway upregulation and DNA repair pathway downregulation. Within cancer islands, % programmed cell death protein 1 expression in CD8+ T cells significantly increased after HIPEC. An exceptional responder (PFS 58 months) demonstrated the highest programmed cell death protein 1 increase. Heat shock proteins comprised the top differentially upregulated genes in HIPEC-treated tumors.
Conclusion: Distinct transcriptomic signatures identify responders to HIPEC, and preclinical model findings are confirmed for the first time in a human cohort.
Trial registration: ClinicalTrials.gov NCT01970722.
Conflict of interest statement
Figures



References
-
- de Bree E, Tsiftsis DD: Experimental and pharmacokinetic studies in intraperitoneal chemotherapy: From laboratory bench to bedside. Recent Results Cancer Res 169:53-73, 2007 - PubMed
-
- Armstrong DK, Bundy B, Wenzel L, et al. : Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34-43, 2006 - PubMed
-
- Mackay HJ, Kohn EC: Intraperitoneal chemotherapy: Hot, timely, and relevant? Cancer 126:5206-5209, 2020 - PubMed
-
- Dellinger TH, Han ES: State of the Science: The role of HIPEC in the treatment of ovarian cancer. Gynecol Oncol 160:364-368, 2021 - PubMed
-
- van Driel WJ, Koole SN, Sikorska K, et al. : Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 378:230-240, 2018 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

