Cell senescence, rapamycin and hyperfunction theory of aging
- PMID: 35358003
- PMCID: PMC9278457
- DOI: 10.1080/15384101.2022.2054636
Cell senescence, rapamycin and hyperfunction theory of aging
Abstract
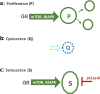
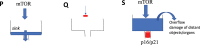
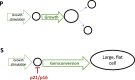
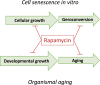
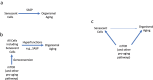
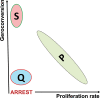
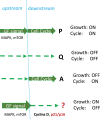
A hallmark of cellular senescence is proliferation-like activity of growth-promoting pathways (such as mTOR and MAPK) in non-proliferating cells. When the cell cycle is arrested, these pathways convert arrest to senescence (geroconversion), rendering cells hypertrophic, beta-Gal-positive and hyperfunctional. The senescence-associated secretory phenotype (SASP) is one of the numerous hyperfunctions. Figuratively, geroconversion is a continuation of growth in non-proliferating cells. Rapamycin, a reversible inhibitor of growth, slows down mTOR-driven geroconversion. Developed two decades ago, this model had accurately predicted that rapamycin must extend life span of animals. However, the notion that senescent cells directly cause organismal aging is oversimplified. Senescent cells contribute to organismal aging but are not strictly required. Cell senescence and organismal aging can be linked indirectly via the same underlying cause, namely hyperfunctional signaling pathways such as mTOR.
Keywords: Senescence; geroconversion; geroscience; gerostatics; healthspan; rapalogs; sirolimus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Serrano M, Lin AW, McCurrach ME, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. - PubMed
-
- Blagosklonny MV. The mitogen-activated protein kinase pathway mediates growth arrest or E1A-dependent apoptosis in SKBR3 human breast cancer cells. Int J Cancer. 1998;78(4):511–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
