Neutrophil extracellular traps regulate ischemic stroke brain injury
- PMID: 35358095
- PMCID: PMC9106355
- DOI: 10.1172/JCI154225
Neutrophil extracellular traps regulate ischemic stroke brain injury
Abstract
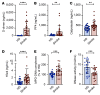
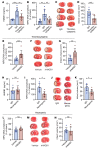
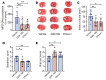
Ischemic stroke prompts a strong inflammatory response, which is associated with exacerbated outcomes. In this study, we investigated mechanistic regulators of neutrophil extracellular trap (NET) formation in stroke and whether they contribute to stroke outcomes. NET-forming neutrophils were found throughout brain tissue of ischemic stroke patients, and elevated plasma NET biomarkers correlated with worse stroke outcomes. Additionally, we observed increased plasma and platelet surface-expressed high-mobility group box 1 (HMGB1) in stroke patients. Mechanistically, platelets were identified as the critical source of HMGB1 that caused NETs in the acute phase of stroke. Depletion of platelets or platelet-specific knockout of HMGB1 significantly reduced plasma HMGB1 and NET levels after stroke, and greatly improved stroke outcomes. We subsequently investigated the therapeutic potential of neonatal NET-inhibitory factor (nNIF) in stroke. Mice treated with nNIF had smaller brain infarcts, improved long-term neurological and motor function, and enhanced survival after stroke. nNIF specifically blocked NET formation without affecting neutrophil recruitment after stroke. Importantly, nNIF also improved stroke outcomes in diabetic and aged mice and was still effective when given 1 hour after stroke onset. These results support a pathological role for NETs in ischemic stroke and warrant further investigation of nNIF for stroke therapy.
Keywords: Hematology; Neutrophils; Platelets; Stroke.
Conflict of interest statement
Figures








References
-
- Virani SS, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

